How Does the TLR4/NF-κB Pathway Contribute?
Table of Contents
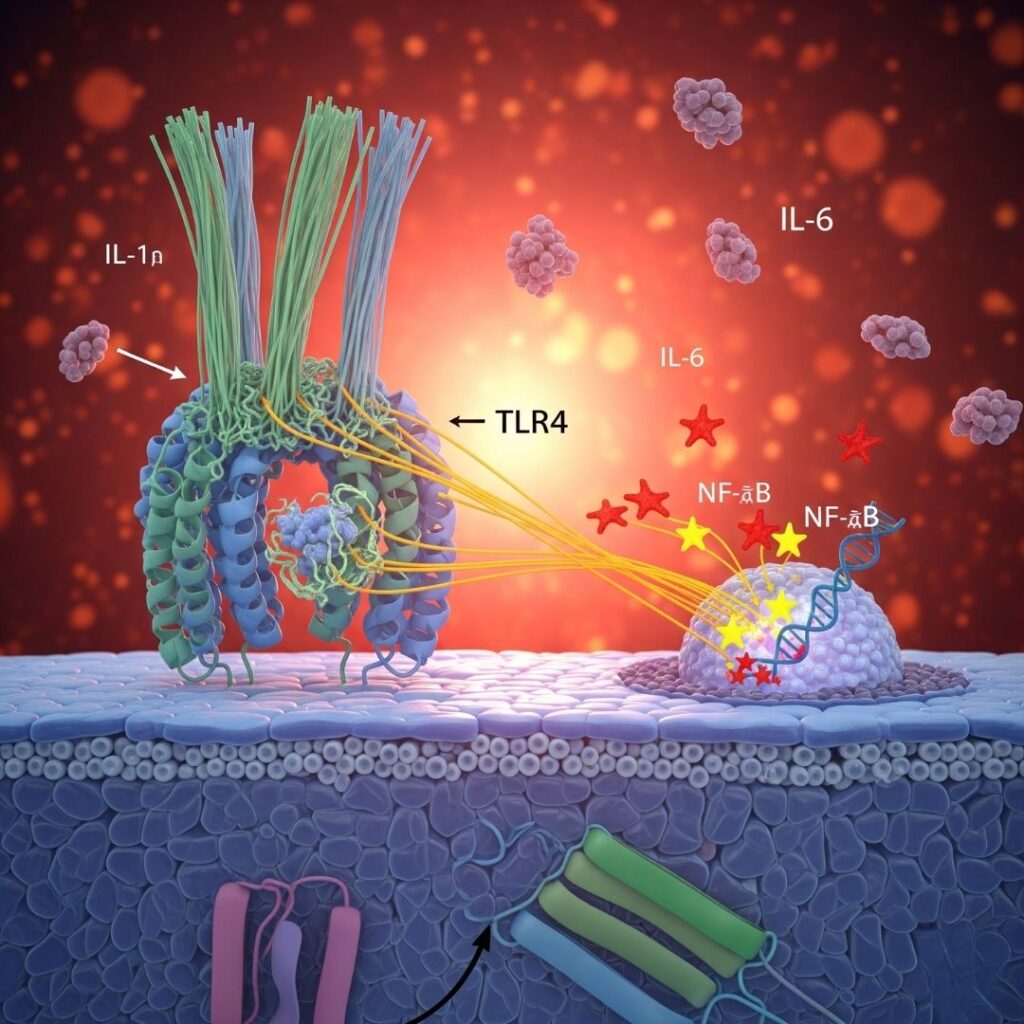
Amyloidosis is a multifaceted and frequently fatal disease characterized by the accumulation of misfolded protein aggregates called amyloid fibrils in tissues and organs. The fibrils disrupt normal cellular function, eventually resulting in organ dysfunction or failure. Of the many signaling pathways involved in amyloidosis-mediated tissue injury, the TLR4/NF-κB signaling pathway is especially important in mediating inflammation and immune responses.
This paper strives to give an in-depth account of the TLR4/NF-κB pathway, its biological role, how it is activated by amyloid fibrils, and how it leads to inflammatory damage in amyloidosis.
Understanding Amyloidosis
Amyloidosis is a collection of diseases where proteins fold in an abnormal manner, then insoluble fibrils that accumulate in tissues are formed. These interfere with cellular function and cause inflammation and tissue damage.
There are a number of different types of amyloidosis, such as:
- AL (Primary) Amyloidosis – due to light chain proteins.
- AA (Secondary) Amyloidosis – secondary to chronic inflammation.
- ATTR Amyloidosis – due to transthyretin protein misfolding.
Although the identity of the fibril-forming protein differs between types, inflammatory signaling seems to be a unifying pathogenic mechanism, usually mediated by the TLR4/NF-κB pathway.
What is the TLR4/NF-κB Pathway?
The TLR4/NF-κB signaling pathway is a part of the innate immune system. It serves as a cellular sensor and responder to pathogenic molecules and internal damage signals (DAMPs—Damage-Associated Molecular Patterns).
- TLR4 (Toll-Like Receptor 4) resides on immune cells such as macrophages and dendritic cells.
- NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) is a transcription factor that controls immune response and inflammation genes.
Upon activation, this pathway induces an inflammatory response, facilitating the release of cytokines, chemokines, and adhesion molecules.
The Toll-Like Receptor 4 (TLR4) Role
TLR4 is a key receptor responsible for detecting both exogenous pathogens (e.g., bacterial lipopolysaccharides) and endogenous molecules such as amyloid fibrils.
TLR4 Functions:
- Detects pattern-associated molecular patterns (PAMPs) and DAMPs
- Activates intracellular signaling through MyD88 or TRIF
- Stimulates downstream signaling pathways, including NF-κB
Expression of TLR4:
- Macrophages
- Microglia (in the brain)
- Renal tubular cells
- Cardiac tissues
In amyloidosis, amyloid fibrils imitate DAMPs and activate TLR4, erroneously suggesting tissue damage or infection and eliciting an immune response.
NF-κB: The Master Regulator of Inflammation
NF-κB is a protein complex present in virtually all animal cells and is a master regulator of immune response. It is present in the cytoplasm in its inactive form bound to IκB proteins.
On activation:
- IκB is broken down.
- NF-κB translocates into the nucleus.
- Binds to DNA promoter regions of pro-inflammatory genes.
Genes Controlled by NF-κB:
- TNF-α
- IL-1β
- IL-6
- ICAM-1
- COX-2
These gene products altogether exacerbate inflammation, leading to both acute immune responses and chronic tissue damage.
Amyloid Fibrils and TLR4 Activation
Recent findings show that amyloid fibrils, particularly Aβ (in Alzheimer’s) and AL fibrils, can directly interact with TLR4 on immune cells.
Mechanisms of Interaction:
- Binding of fibrils to TLR4
- Recruitment of co-receptors such as MD-2 and CD14
- Induce conformational changes and activate downstream signaling
Amyloid fibrils are danger signals, functioning like pathogens and tricking the immune system into a chronic state of inflammation.
Inflammatory Cascade: TLR4 to NF-κB
Here’s a brief overview of amyloid fibril activation of the inflammatory signaling cascade:
- Recognition: Binding of amyloid fibrils to TLR4.
- Recruitment of Adaptors: MyD88 and TRIF are recruited.
- Activation of Kinases: IRAK1, TRAF6, and TAK1 are activated.
- Activation of IKK Complex: Phosphorylates IκB and tags it for degradation.
- NF-κB Translocation: NF-κB enters the nucleus.
- Transcription of Genes: Cytokine genes are expressed.
- Inflammation: Cytokines are secreted, leading to immune cell recruitment.
Key Cytokines and Chemokines Released
The TLR4/NF-κB pathway contributes to the generation of inflammatory mediators that drive tissue damage and enhance amyloid-induced toxicity.
Major Cytokines:
- TNF-α (Tumor Necrosis Factor-alpha): Enhances vascular permeability, causes apoptosis.
- IL-1β: Induces fever, stimulates endothelial cells.
- IL-6: Stimulates production of acute-phase proteins in the liver.
- MCP-1 (Monocyte Chemoattractant Protein-1): Attracts monocytes to sites of inflammation.
These cytokines recruit immune cells to inflamed tissues, tending to increase damage rather than cure disease.
Tissue Damage and Organ Involvement
The TLR4/NF-κB pathway plays a role in multi-organ damage in amyloidosis due to prolonged and uncontrolled inflammation.
Organs Frequently Involved:
- Heart: Infiltration causes restrictive cardiomyopathy.
- Kidneys: Inflammation is a cause of nephrotic syndrome.
- Liver: Hepatomegaly and dysfunction secondary to immune activation.
- Nervous System: Neuroinflammation is a characteristic in ATTR and Alzheimer’s-related amyloidosis.
Inflammation also disturbs normal organ architecture, compromises cellular functions, and aggravates disease progression.
Experimental Evidence and Research Findings
Many preclinical studies and clinical observations validate the pivotal role of the TLR4/NF-κB pathway in amyloidosis:
- Animal Models: Mice that are injected with amyloid fibrils exhibit increased TLR4 and NF-κB expression.
- Inhibitor Studies: TLR4 antagonists decrease levels of cytokines and enhance survival in models of amyloidosis.
- Human Biopsies: Increased expression of TLR4 and NF-κB markers in cardiac and renal tissues of amyloidosis patients.
These findings identify this pathway as a key therapeutic target to modulate inflammation in amyloidosis.
Therapeutic Implications and Drug Targets
With its key position in inflammation, modulation of the TLR4/NF-κB pathway represents a potential strategy for the treatment of amyloidosis.
Emerging Therapies:
- TLR4 Antagonists – e.g., TAK-242, Eritoran
- NF-κB Inhibitors – e.g., Bortezomib (also in AL amyloidosis), Parthenolide
- Cytokine Blockers – e.g., Anti-TNF agents, IL-1 receptor antagonists
- Small Molecule Inhibitors – Block IKK complex or MyD88 pathways
- RNA-Based Therapies – siRNA to knockdown NF-κB subunits or TLR4
Complementary Approaches:
- Antioxidants – Minimize oxidative stress that exacerbates NF-κB activation.
- Lifestyle Interventions – Nutrition and physical activity to restrict chronic inflammation.
Conclusion
The TLR4/NF-κB pathway is a key inflammatory pathway in amyloidosis, initiated by the abnormal accumulation of amyloid fibrils. Activation of the pathway initiates an immune response cascade, that releases pro-inflammatory cytokines with further tissue destruction and enhanced disease progression.
Recognition and targeting this pathway not only raises our understanding of amyloid pathophysiology but also provides new treatment avenues, particularly in the early or moderate phases of the disease.
With advancing research, treatments designed to block TLR4 or NF-κB are highly promising in reducing patients’ burden from amyloidosis and enhancing patient outcome.
FAQs
1. Can amyloid fibrils directly activate the immune system?
Yes, they can. Amyloid fibrils function as danger signals (DAMPs) and stimulate immune pathways such as TLR4 and induce inflammation.
2. Why is NF-κB a key player in amyloidosis?
NF-κB controls genes that govern inflammation, immune response, and cell survival—all essential in the development of amyloidosis.
3. Are there any medications focused on the TLR4/NF-κB pathway in amyloidosis?
Certain investigational medications such as TLR4 antagonists and proteasome inhibitors hold potential in the modulation of this pathway.
4. What is the role of chronic inflammation in amyloidosis?
Chronic inflammation exacerbates tissue damage, encourages secondary amyloid deposition, and enhances symptoms of disease.
5. How is this pathway further researched?
Animal models, patient biopsies, and cellular assays continue to be fundamental tools for the evaluation of TLR4/NF-κB signaling’s effect and modulation.

