Case Report: Transthoracic Echocardiogram Demonstrates Left Ventricular Hypertrophy Consistent with Infiltrative Process in a 61-Year-Old Patient
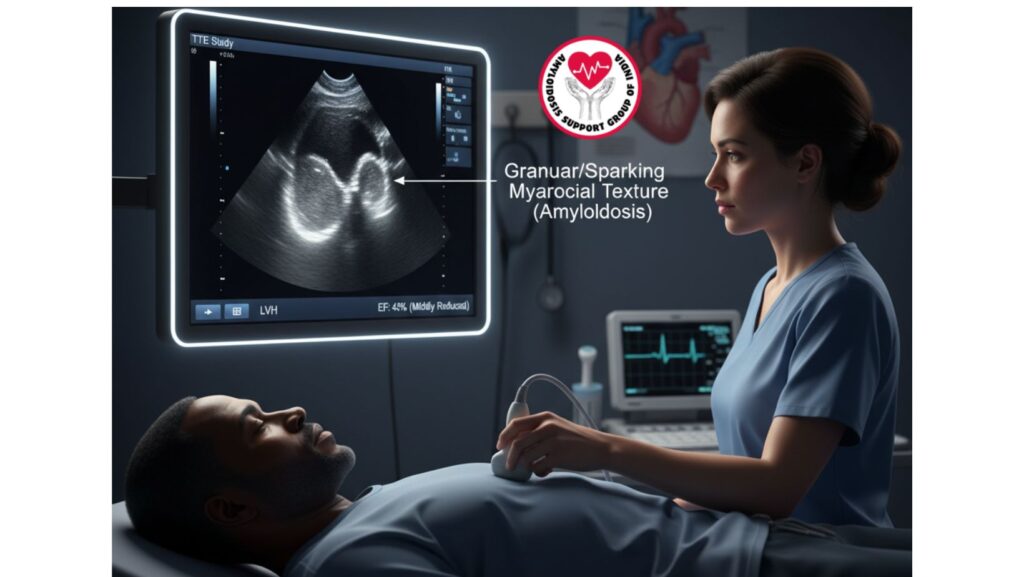
Table of Contents
Introduction
Cardiac imaging assumes a central role in the diagnosis of underlying structural and functional derangements in patients with systemic disease. Infiltrative cardiomyopathies, like amyloidosis, sarcoidosis, and hemochromatosis, characteristically yield subtle findings on transthoracic echocardiography (TTE) prior to progression to overt clinical heart failure. This case presentation addresses a 61-year-old African-American man whose TTE demonstrated left ventricular hypertrophy (LVH) suspicious for an infiltrative process, highlighting the significance of cardiac assessment in systemic disease presentation.
Patient Background
The patient, a 61-year-old African-American man, had a subacute history of abdominal distension, lower extremity swelling, and portal hypertension signs. Initial laboratory results were hypoalbuminemia (2.1 g/dL), elevated liver enzymes (AP 718 IU/L, AST 158 IU/L, ALT 119 IU/L), and proteinuria of nephrotic range. Viral and autoimmune panels were both normal, and abdominal CT showed ascites with probable nodular liver changes without hepatosplenomegaly.
Based on the syndrome of symptoms and ascites evaluation, the patient was suspected to have a systemic process, and further cardiac workup was indicated.
Clinical Reasoning for Echocardiography
Patients with systemic amyloidosis or other infiltrative diseases typically have multiorgan involvement manifested as:
- Renal manifestations: nephrotic syndrome and proteinuria
- Hepatic manifestations: hepatomegaly or abnormal enzymes
- Cardiac manifestations: restrictive cardiomyopathy, arrhythmias, or conduction disorders
Transthoracic echocardiography (TTE) was done to evaluate for cardiac structural and functional alterations that could account for systemic involvement.
Echocardiographic Findings
The TTE showed concentric left ventricular hypertrophy. Although LVH is a common finding with hypertension, in this patient the appearance was unusual:
- Symmetrical thickening of the LV walls
- Normal ejection fraction
- “Sparkling” or granular appearance of the myocardium (a classic but not invariably present sign of amyloidosis)
- Diastolic dysfunction with restrictive filling pattern
These findings were suspicious for an infiltrative process like amyloidosis, and not hypertensive heart disease per se.
Differential Diagnosis of LVH with Infiltrative Features
- Cardiac Amyloidosis – Amyloid fibril deposition within the myocardium resulting in restrictive physiology.
- Hypertensive Heart Disease – Chronic hypertension leading to LVH; less likely considering systemic features.
- Hypertrophic Cardiomyopathy (HCM) – Typically asymmetric, not concentric.
- Sarcoidosis – May resemble infiltrative cardiomyopathy but usually has conduction defects and granulomatous inflammation.
- Hemochromatosis – Iron overload cardiomyopathy; serum iron tests would be diagnostic.
Echocardiographic Role in Infiltrative Cardiomyopathy
Echocardiography is still the first-line non-invasive imaging modality in the evaluation of infiltrative cardiomyopathies:
- Reveals LVH out of proportion to blood pressure
- Tests diastolic dysfunction
- Picks up pericardial effusions, a feature of amyloidosis
- Gives a hint towards pursuing advanced imaging like cardiac MRI or nuclear scintigraphy
Clinical Significance of Findings
The finding of concentric LVH with suspected infiltrative features in the context of systemic disease strongly suggests cardiac amyloidosis. This is an important diagnosis because:
- It affects prognosis – cardiac involvement is a key determinant of survival in amyloidosis.
- It affects treatment plans – conventional heart failure treatment might not work.
- It requires multidisciplinary treatment, such as cardiology, hematology, and nephrology.
Correlation with Patient’s Systemic Findings
The echocardiographic diagnosis correlates well with the patient’s systemic presentation:
- Nephrotic syndrome → Renal amyloid deposits
- Hypoalbuminemia with ascites → Hepatic and renal involvement
- Concentric LVH on TTE → Cardiac amyloidosis suspicion
Combined, this forms a solid foundation for systemic amyloidosis with cardiac involvement.
Management Approach
1. Further Diagnostic Workup
- Cardiac MRI – to characterize myocardial tissue
- 99mTc-PYP scan (nuclear scintigraphy) – for transthyretin amyloidosis (ATTR)
- Endomyocardial or organ biopsy – gold standard for confirmation of amyloid
- Serum and urine immunofixation electrophoresis – to discriminate AL (light-chain) amyloidosis from ATTR
2. Medical Management
- Diuretics for fluid overload
- Exclude beta-blockers and calcium channel blockers (worse tolerance in amyloidosis)
- Type-specific targeted therapy:
- AL amyloidosis – chemotherapy, autologous stem cell transplant
- ATTR amyloidosis – tafamidis or experimental therapies
3. Supportive Care
- Restriction of salt to manage ascites and edema
- Frequent monitoring of renal and cardiac function
- Multidisciplinary follow-up
Prognosis
Infiltrative cardiomyopathy secondary to amyloidosis has an unpredictable prognosis based on the type
- AL amyloidosis – unfavorable prognosis if left untreated, with median survival <1 year since the onset of heart failure.
- ATTR amyloidosis – more gradual development, with survival from 3–10 years based on the availability of therapy.
Early detection by echocardiography is critical for better prognosis.
Conclusion
This case underscores the significance of echocardiography in systemic diseases. The identification of left ventricular hypertrophy with infiltrative features was an important diagnostic hint towards systemic amyloidosis with cardiac involvement. Early detection of such findings can direct further evaluation and lead to proper management, probably changing the patient’s prognosis.

