Nephrotic Range Proteinuria in AL Amyloidosis: Clinical Significance and Renal Involvement
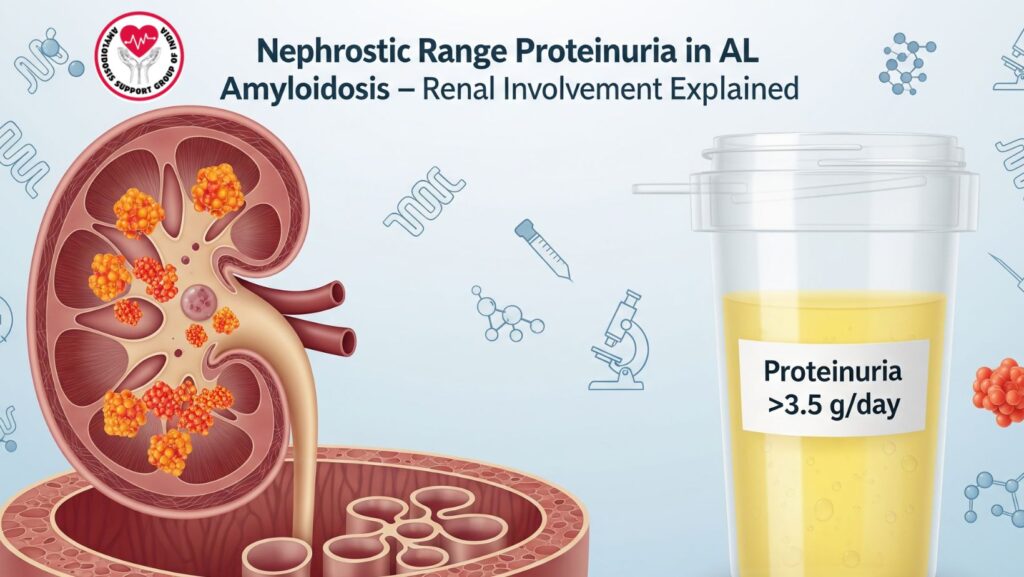
Table of Contents
Introduction
Amyloidosis is a systemic disorder involving extracellular deposition of aberrantly folded proteins, resulting in progressive organ injury. Of its subtypes, primary light-chain (AL) amyloidosis is the most prevalent, being caused by monoclonal plasma cell dyscrasias. Kidney disease is one of the most common and clinically relevant presentations. One of the classic indications of kidney injury in AL amyloidosis is nephrotic range proteinuria, a state that indicates advanced disease and prognostic significance.
This article discusses the clinical importance of nephrotic range proteinuria in AL amyloidosis based on its pathophysiology, workup for diagnosis, course, therapeutic approaches, and influence on outcome.
1. Overview of AL Amyloidosis
- Amyloidosis definition and classification.
- Pathogenesis: clonal plasma cell production of aberrant light chains.
- Organ involvement patterns: kidney, heart, liver, gastrointestinal tract, nervous system.
- Role of early diagnosis in enhancing survival.
2. Renal Involvement in AL Amyloidosis
- Frequency: kidneys are affected in 50–70% of AL amyloidosis.
- Common presentations: proteinuria, nephrotic syndrome, progressive renal failure.
- Progression to end-stage renal disease (ESRD) if left untreated.
3. Nephrotic Range Proteinuria: Definition and Clinical Significance
- Definition: proteinuria >3.5 g/day.
- Clinical presentation: edema, hypoalbuminemia, hyperlipidemia.
- Pathophysiologic basis: amyloid fibril deposition in glomeruli → glomerular damage.
- Why it is a red flag for systemic renal amyloidosis.
4. Pathophysiology of Proteinuria in AL Amyloidosis
- Amyloid deposits in glomerular basement membranes.
- Integrity loss of podocytes and disruption of filtration barrier.
- Course progression from microalbuminuria → overt proteinuria → nephrotic syndrome.
- Correlation with declining glomerular filtration rate (GFR).
5. Diagnostic Workup
- Urinalysis: detection of proteinuria, microscopic hematuria.
- Quantification: 24-hour urine collection or spot urine protein-to-creatinine ratio.
- Serologic tests: serum protein electrophoresis (SPEP), immunofixation, serum free light chains.
- Renal biopsy: Congo red staining to establish amyloid deposits.
- Use of mass spectrometry in amyloid typing.
6. Clinical Implications of Nephrotic Range Proteinuria
- Indicator of renal amyloidosis.
- Forecasts renal progression to ESRD.
- Linked with poor prognosis when in combination with cardiac involvement.
- Direct effect on quality of life due to edema, fatigue, malnutrition.
7. Prognostic Impact
- Patients with nephrotic syndrome develop renal failure more quickly.
- Poorer overall survival than amyloidosis that is not associated with kidney involvement.
- Increased rates of cardiovascular complications secondary to volume overload.
- Prognosis heavily dependent upon hematologic response to treatment.
8. Management Strategies
a) Supportive Care
- Salt restriction and diuretics for edema.
- Statins for hyperlipidemia.
- Anticoagulation in selected patients (risk of thromboembolism).
b) Disease-Modifying Therapy
- Plasma cell–directed therapies (bortezomib, cyclophosphamide, dexamethasone).
- Autologous stem cell transplantation (ASCT) in appropriate patients.
- New drugs: daratumumab, venetoclax (for certain mutations).
c) Renal Replacement Therapy
- Dialysis in ESRD.
- Kidney transplant in carefully selected patients who attain hematologic remission.
9. Case Study Perspective
- The patient was 61 years old and had nephrotic range proteinuria.
- This revealed renal amyloid involvement in addition to hepatic and cardiac disease.
- Was a factor in ineligibility for liver transplant.
- Emphasized the systemic and progressive nature of AL amyloidosis.
10. Future Directions in Management
- Progress in targeted therapies against amyloid fibrils.
- Function of combination regimens in maintaining renal function.
- Clinical trials investigating new monoclonal antibodies and gene therapies.
- Significance of multidisciplinary care in enhancing survival.
Conclusion
Nephrotic range proteinuria is more than a laboratory result in AL amyloidosis—it is an indicator of renal involvement, disease severity, and adverse prognosis. Its detection should lead to vigorous diagnostic investigation and prompt systemic treatment initiation. For those with multi-organ involvement, nephrotic syndrome is a clinical turning point in decision making, including transplant candidacy and supportive care management.

