Anselamimab: Investigational Monoclonal Antibody
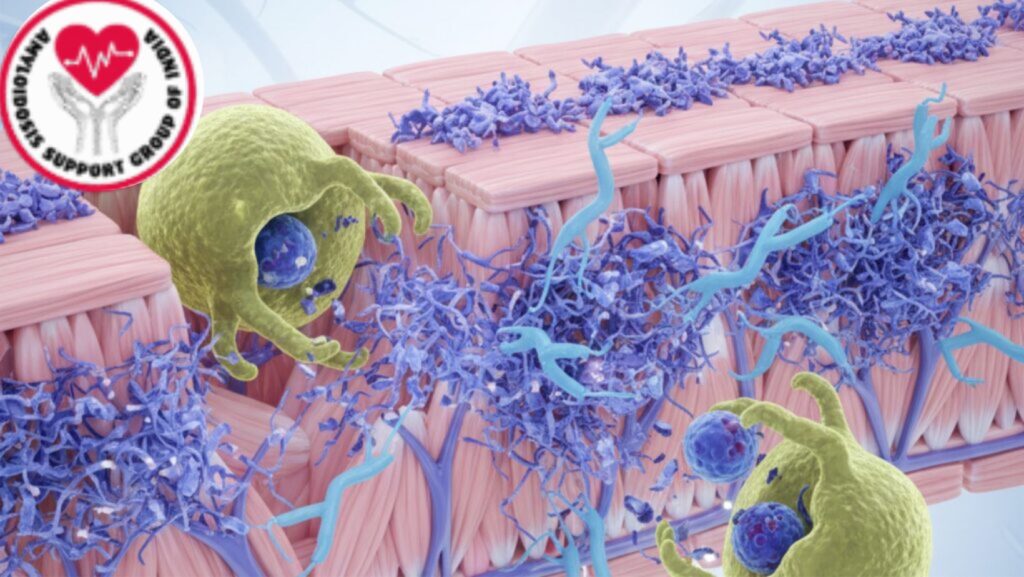
Table of Contents
Introduction
Systemic amyloidosis is a progressive and life-threatening condition due to the misfolding and deposition of amyloid fibrils in organs such as the heart, kidneys, liver, and nervous system. Of its subtypes, light-chain (AL) amyloidosis is the most common, and it is linked with unfavorable survival if left untreated.
Whereas traditional therapies aim at reducing production of precursor protein (e.g., plasma cell–targeted therapies in AL amyloidosis), amyloid deposits themselves are the main contributor to organ dysfunction. Because of this, the discovery of anti-amyloid fibril therapies has emerged as a promising area of research.
Anselamimab is an investigational monoclonal antibody targeted to amyloid fibrils, designed to increase their clearance and enhance organ function. Through the direct binding to deposited fibrils, anselamimab can potentially cause their removal via immune-mediated processes.
In this article, an in-depth review of:
- The history of amyloidosis and the existing treatment deficit
- Anslemamab’s mechanism of action
- Preclinical and clinical data to date
- Its potential for transforming the treatment paradigm for amyloidosis
- Future directions and challenges
Section 1: Amyloidosis Overview
1.1 Definition and Classification
Amyloidosis is a group of diseases involving extracellular deposition of insoluble fibrils made up of misfolded proteins.
- AL Amyloidosis: Of immunoglobulin light chain origin (κ or λ)
- ATTR Amyloidosis: Due to transthyretin misfolding (hereditary or wild-type)
- AA Amyloidosis: Can result from serum amyloid A in chronic inflammation
- Rare forms: e.g., gelsolin, apolipoprotein A-I, β2-microglobulin
1.2 Organ Involvement
Amyloid fibrils aggregate in various organs, resulting in:
- Heart → restrictive cardiomyopathy, arrhythmias
- Kidney → nephrotic syndrome, renal failure
- Liver → hepatomegaly, cholestasis
- Nervous system → neuropathy, autonomic dysfunction
1.3 Limitations of Current Therapies
Current therapy aims to stop amyloid synthesis:
- Chemotherapy regimens (e.g., bortezomib, daratumumab-based therapies)
- Stem cell transplantation
- TTR stabilizers and silencers in ATTR
Yet, no licensed therapy directly removes existing amyloid deposits. This is where anselamimab comes in as a new therapeutic approach.
Section 2: Monoclonal Antibodies in Amyloidosis
2.1 Rationale for Antibody Therapy
Monoclonal antibodies (mAbs) transformed oncology and immunology. Application in amyloidosis relies on the fact that:
- Amyloid fibrils present distinctive neoepitopes that are not found on native precursor proteins.
- Antibodies can bind to these epitopes and **activate immune clearance mechanisms”.
2.2 Previous Attempts
Several anti-amyloid antibodies have been generated:
- Birtamimab (NEOD001) – early promise, but Phase III trial failure
- CAEL-101 – trials underway in AL amyloidosis
- PRX004 – transthyretin amyloid (ATTR amyloidosis) targeted
These attempts showed that it was possible, opening the way for anselamimab.
Section 3: What is Anselamimab?
3.1 Definition
Anselamimab is an investigational monoclonal antibody that is intended to:
- Bind to amyloid fibrils deposited in tissues
- Facilitate phagocytic clearance by immune cells
- Decrease amyloid burden and restore organ function
3.2 Molecular Design
- Humanized monoclonal antibody (IgG isotype)
- Designed for high-affinity binding to amyloid fibril epitopes
- Fc-region optimized to activate immune effector cells
3.3 Differentiating Features
- Wider spectrum of binding to amyloid fibrils than antecedents
- Possibly reduced immunogenicity
- Formulated for organ-directed functional improvement, rather than hematologic remission
Section 4: Mechanism of Action
Anselamimab’s mechanism can be outlined in three steps:
- Amyloid Fibril Binding
- Binds to amyloid-specific conformational epitopes
- Fails to bind soluble precursors in the bloodstream, preventing off-targeting
- Immune Recruitment
- Recruits Fcγ receptors on macrophages and other effector cells
- Initiates antibody-dependent cellular phagocytosis (ADCP)
- Amyloid Clearance and Organ Restoration
- Progressive clearance of amyloid fibrils from organs
- Results in enhanced organ function (e.g., cardiac output, renal filtration)
Section 5: Preclinical Evidence
5.1 In Vitro Studies
- Established selective binding to AL amyloid fibrils
- Established immune-mediated clearance mechanisms
5.2 Animal Models
- Demonstrated decrease in amyloid deposits in murine models
- Improvement of cardiac and renal biomarkers
5.3 Translational Relevance
- Strong biological rationale for clinical development
- Supports existing trial designs
Section 6: Clinical Development of Anselamimab
6.1 Phase I Trials
- Purpose: Safety, tolerability, pharmacokinetics
- Results: Well-tolerated, dose-dependent fibril targeting
6.2 Phase II/III Programs
- Population: Patients with AL amyloidosis and organ involvement
- Outcomes:
- Primary: Organ response (cardiac, renal)
- Secondary: Survival, quality of life, hematologic response
- Ongoing investigations in multi-center trials
6.3 Safety Profile
- Generally manageable infusion-related toxicities
- No unexpected safety issues to date
Section 7: Clinical Significance
7.1 Meeting Unmet Needs
- Explicitly addresses amyloid deposits not addressed by standard therapies
- Could revolutionize organ prognosis in advanced AL amyloidosis
7.2 Synergistic Role with Current Treatments
- Employed in combination with plasma cell–targeted therapies
- Could permit accelerated organ recovery
7.3 Potential Across Amyloidosis Subtypes
- While only investigated extensively in AL amyloidosis, mechanism predicts application in:
- ATTR amyloidosis
- AA amyloidosis
Section 8: Challenges and Future Outlook
8.1 Challenges
- Providing demonstrated survival benefit in clinical trials
- Patient accessibility and affordability
- Management of potential immune-related adverse effects
8.2 Future Directions
- Combination with daratumumab-based regimens
- Preemptive intervention for high-risk AL amyloidosis
- Expansion to ATTR and other amyloidosis subtypes
- Use in minimal residual disease (MRD) cases
Section 9: FAQs
Q1. What is different about anselamimab compared with earlier antibodies?
Its design enhances specificity, minimizes immunogenicity, and prioritizes organ recovery.
Q2. Is anselamimab approved?
No, it is investigational and still in clinical evaluation.
Q3. Is anselamimab a cure for amyloidosis?
Not a cure, but potentially could dramatically improve survival and organ outcomes when used with best supportive care.
Q4. For whom is anselamimab available?
At present, only for patients participating in clinical trials.
Q5. How is the approval outlook?
If benefit is shown with ongoing Phase II/III trials, it could be a breakthrough drug for AL amyloidosis.
Conclusion
Anselamimab is a paradigm change in amyloidosis treatment. In contrast to current therapies that merely dampen precursor protein production, it truly targets amyloid fibrils, in an attempt to diminish deposits and recover organ function.
If clinical trials substantiate its safety and effectiveness, anselamimab may be the first broadly accepted anti-amyloid fibril therapy, providing new promise for individuals with AL amyloidosis and perhaps other systemic amyloidosis conditions.
Its odyssey echoes the increasing potential of precision immunotherapy in uncommon protein misfolding disorders and highlights the significance of combining organ-directed therapies with hematologic interventions.

