Introduction
Amyloid proteins are misfolded protein aggregates that are responsible for a category of severe health conditions referred to as amyloidosis. The proteins may accumulate within tissues and organs, interfering with normal functions and resulting in various diseases. Knowing what amyloid proteins are, how they develop, and what effect they have on human health is important for spreading awareness about these diseases.
In this article, we will discuss what amyloid proteins are, how they are produced, their biological significance, the diseases that are caused by them, and recent research trends. Whether you are a patient, caregiver, physician, or are simply curious, this in-depth guide will acquaint you with amyloid proteins.
1. What Are Amyloid Proteins?
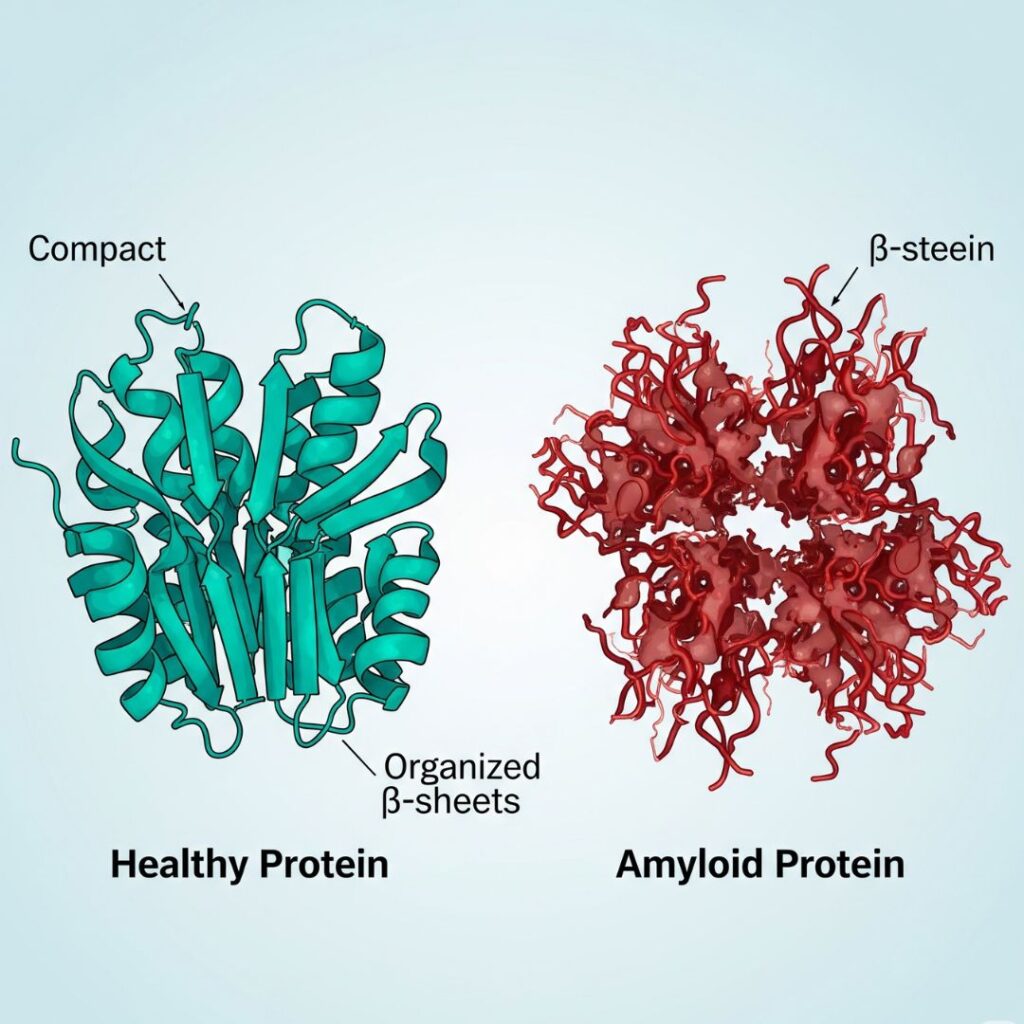
Amyloid proteins are defective proteins that self-aggregate to form insoluble fibrils and get deposited in tissues. In contrast to regular proteins that fold into characteristic three-dimensional structures to carry out essential cellular activity, amyloid proteins are folded abnormally and take on beta-sheet-dominant structures.
The misfolded proteins aggregate to become fibrils, which accumulate in the form of plaques or deposits in tissues. Such deposits are poisonous and can compromise the functioning of essential organs.
Key Features of Amyloid Proteins:
- Misfolded structure
- Beta-sheet conformational rich
- Resistant to proteolysis (hard to degrade)
- Water-insoluble
- Fibrillar aggregate formation capability
2. How Do Amyloid Proteins Form?
Amyloid proteins tend to form when the normal protein folding process fails. This is how the process generally occurs:
- Protein Synthesis: Proteins are synthesized from amino acids within the ribosomes.
- Folding: Proteins that have just been synthesized fold into defined 3D structures required for their function.
- Misfolding: Because of gene mutation, environmental stress, or aging, proteins can misfold.
- Aggregation: Misfolded proteins tend to aggregate, forming oligomers and eventually fibrils.
- Deposition: Such fibrils deposit as amyloid deposits in tissues and organs.
The conversion from a soluble monomeric protein into an insoluble amyloid fibril is a characteristic of most amyloid diseases.
3. Why are Amyloid Proteins Toxic?
Amyloid proteins are toxic because they disrupt normal cellular and organ function. They can:
- Impede cellular communication
- Harm cellular membranes
- Trigger inflammation
- Cause tissue stiffness
- Result in organ failure
If amyloid deposits form, they tend to be hard to remove and will continue to expand, leading to progressive disease.
4. Diseases Caused by Amyloid Proteins
There are a few diseases associated with amyloid protein deposits. Such diseases can be localized (one organ involved) or systemic (many organs involved).
a. Alzheimer’s Disease
Alzheimer’s is one of the most recognized conditions with amyloid. Amyloid-beta plaques accumulating in the brain interfere with neural communication and cause memory loss and cognitive decline.
b. Parkinson’s Disease
Characterized by alpha-synuclein amyloid deposits known as Lewy bodies, Parkinson’s impacts motor control and produces tremors and stiffness.
c. Systemic Amyloidosis
This category consists of:
- AL (Light Chain) Amyloidosis: Results from defective antibodies.
- AA (Serum Amyloid A) Amyloidosis: Usually the consequence of chronic inflammatory diseases.
- ATTR (Transthyretin) Amyloidosis: May be inherited or age-associated.
d. Huntington’s Disease
Mutant huntingtin protein creates amyloid-like inclusions, resulting in neurodegeneration.
e. Type 2 Diabetes
Amylin, a hormone co-secreted with insulin, may deposit as amyloid in the pancreas and lead to beta-cell dysfunction.
5. Diagnosis of Amyloid-Related Diseases
Common Diagnostic Techniques:
- Biopsy: Microscopic examination of tissue samples.
- Congo Red Staining: Amyloid stains apple-green under polarized light.
- Immunohistochemistry: Determines the amyloid protein type.
- Blood and Urine Tests: Identify abnormal proteins.
- Imaging Modalities: MRI, PET scans, and echocardiography for organ evaluation.
6. Existing Treatments and Therapies
There is no single cure for amyloidosis, but treatments seek to lower amyloid production, control symptoms, and enhance quality of life.
Treatment Approaches:
- Medications: Tafamidis, Patisiran, Inotersen, Doxycycline
- Chemotherapy: For AL amyloidosis
- Liver or Heart Transplant: For advanced organ damage
- Gene Therapy: New therapies for genetic mutations
- Lifestyle Modifications: Diet, exercise, and management of underlying conditions
7. Ongoing Research and Future Directions
Researchers are working actively on understanding the mechanism of amyloid formation and prevention. Some promising areas are:
- Immunotherapy: Employing antibodies to attack and eliminate amyloid plaques
- Small Molecule Inhibitors: Inhibit amyloid aggregation
- RNA-based Therapies: Suppress genes that manufacture toxic proteins
- Artificial Intelligence: To simulate protein folding and create improved drugs
8. Living with Amyloidosis
It can be difficult living with an amyloid-related disease, but if taken care of and supported accordingly, patients can lead a satisfactory quality of life.
Tips for Patients and Families:
- Educate yourself about the condition
- Take regular follow-ups with specialists
- Adhere to treatment protocols
- Seek psychological and emotional support
- Participate in support groups like AmyloidosisSupport and Rdssdfsocial
9. FAQs
Q1: Are amyloid proteins always harmful?
No. Amyloids are naturally found in some instances, but abnormally accumulated amyloids are dangerous.
Q2: Is amyloidosis curable?
No, not entirely, but medication can slow it and control the symptoms.
Q3: Is amyloidosis hereditary?
Part of it like ATTR is inherited, whereas the other type like AL is not.
Q4: How rare is amyloidosis?
Amyloidosis is a rare disease but can be underdiagnosed since it presents nonspecifically.
Q5: What is amyloidosis life expectancy?
That varies with the type, severity, and timely diagnosis. Some types have a better prognosis than others.
Conclusion
Misfolded amyloid proteins deposited within the body cause severe health hazards and result in complicated diseases such as Alzheimer’s, Parkinson’s, and systemic amyloidosis. Timely diagnosis, patient education, and availability of treatments are crucial for an effective management of such conditions.
At AmyloidosisSupport, we aim to deliver accurate, current, and accessible information to patients and their families. We are convinced that informed support can have a dramatic impact on people’s lives.

