Introduction
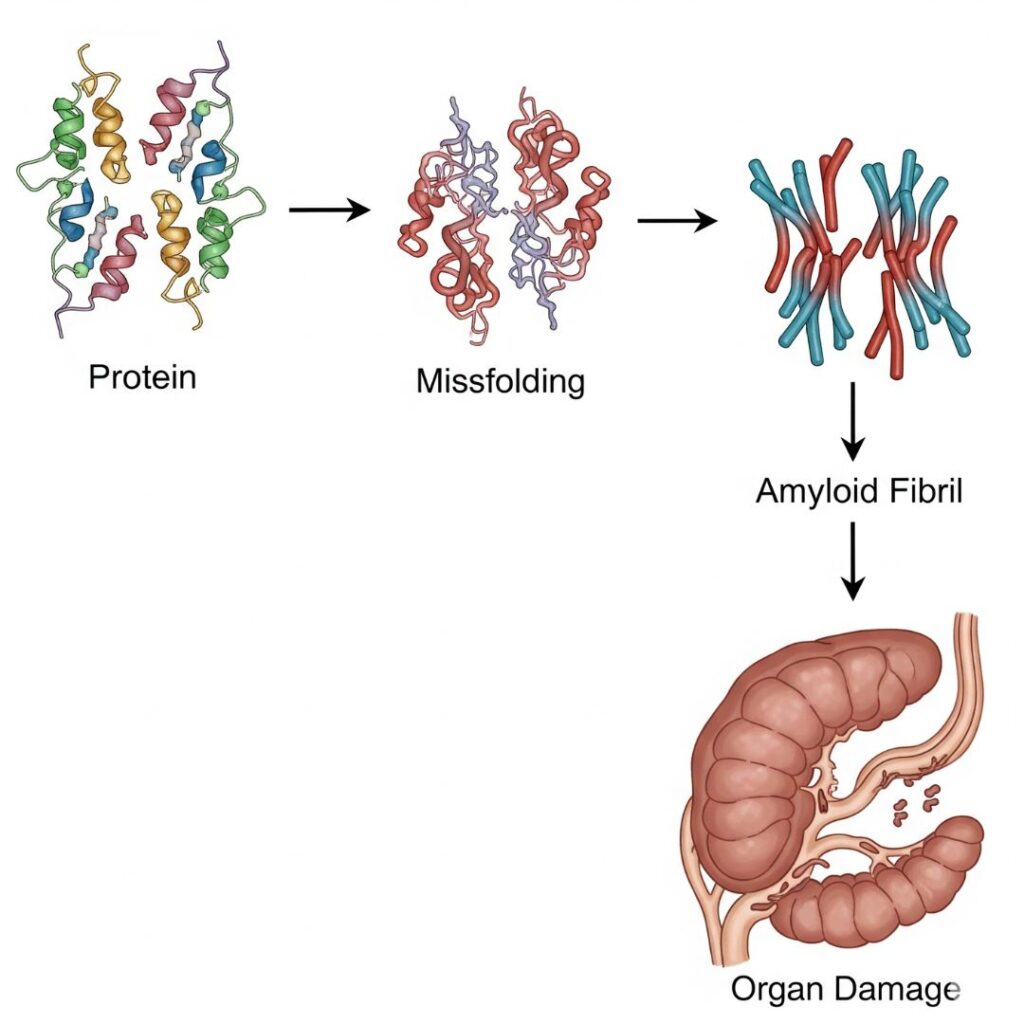
Amyloidosis is a difficult and sometimes obscure disease that belongs to an unusual group of disorders called protein misfolding disorders. Protein misfolding disorders are defined by the inappropriate folding of proteins and the subsequent development of insoluble aggregates that interfere with normal biological processes. In amyloidosis, the protein aggregates are referred to as amyloid fibrils, which accumulate in tissues and organs and lead to progressive damage.
In this blog post, we will discuss the molecular mechanism of amyloidosis, define what protein misfolding disorders are, and in detail reason why amyloidosis is to be classified as such. We will also briefly go through the implications of protein misfolding in human health, ongoing research, and the strategies currently being developed to treat this class of diseases.
1. Understanding Proteins and Their Functions
Proteins are vital molecules consisting of lengthy chains of amino acids. These chains fold into precise three-dimensional structures so that they can perform important cellular functions such as:
- Enzyme reactions
- Cellular signaling
- Molecule transport
- Immune function
- Structural support
The functionality of a protein solely relies on its proper folding. This complex folding process is directed by the amino acid sequence of the protein and the cellular environment.
2. What is Protein Misfolding?
Protein misfolding refers to a situation where a recently made protein does not take on its desired three-dimensional structure. Rather, it folds into a deviant shape that makes it dysfunctional or even toxic.
Causes of Misfolding:
- Genetic mutations
- Environmental stress
- Aging
- Oxidative damage
- Errors in protein synthesis
Under normal circumstances, cells possess quality control mechanisms (such as chaperone proteins and proteasomes) to repair or destroy misfolded proteins. But when the mechanisms malfunction or become overburdened, misfolded proteins start to accumulate.
3. Amyloid Fibrils in Amyloidosis
In amyloidosis, some proteins misfold and form amyloid fibrils, which are insoluble and resistant to breakdown. These fibrils accumulate in organs like the heart, kidneys, liver, and nervous system, compromising their structure and function.
Most Important Features of Amyloid Fibrils:
- Rich content of beta-sheets
- Insolubility in water
- Strong resistance to proteolysis
- Propensity to aggregate
This pathological deposition of amyloid fibrils defines amyloidosis and categorizes it as a protein misfolding disorder.
4. Why Is Amyloidosis a Protein Misfolding Disorder?
Amyloidosis is a protein misfolding disorder because:
- The disease begins with protein misfolding: A normally soluble and functional protein misfolds.
- Misfolded proteins aggregate: The proteins aggregate to create toxic oligomers and ultimately fibrils.
- Fibrils deposit in tissues: Amyloid deposition interferes with normal organ function.
- Cellular systems fail to degrade these fibrils: Cellular defense systems cannot remove these aggregates.
All of this, from misfolding to aggregation to tissue deposition, is the signature of a misfolding disorder.
5. Types of Amyloidosis and Their Misfolded Proteins
There are various types of amyloidosis, each of which is linked with a different misfolded protein:
a. AL (Light Chain) Amyloidosis
- Due to misfolded light chains in abnormal plasma cells
- Frequent in multiple myeloma
b. AA (Serum Amyloid A) Amyloidosis
- Due to misfolded serum amyloid protein
- Associated with chronic inflammation and infections
c. ATTR (Transthyretin) Amyloidosis
- Due to misfolded transthyretin protein
- Can be inherited or age-dependent (wild-type)
d. Beta-2 Microglobulin Amyloidosis
- Present in dialysis patients
- Accumulation of misfolded beta-2 microglobulin protein
All of these disorders include a different protein, yet they all follow the common route of protein misfolding and protein aggregation.
6. Protein Misfolding in Other Diseases
Amyloidosis is a member of a larger category of neurodegenerative and systemic diseases due to protein misfolding, which includes:
- Alzheimer’s disease: Amyloid-beta and tau protein misfolding
- Parkinson’s disease: Alpha-synuclein misfolding
- Huntington’s disease: Mutant huntingtin protein aggregation
- Prion diseases: Misfolded prion proteins
The common mechanism in these disorders underlines the significance of protein folding in ensuring health and avoiding disease.
7. Diagnostic Implications of Protein Misfolding
Diagnosis is facilitated by understanding amyloidosis as a protein misfolding disorder:
Diagnostic Techniques:
- Tissue biopsy with Congo red staining: Reveals apple-green birefringence under polarized light
- Mass spectrometry and immunohistochemistry: Detect particular amyloid proteins
- Genetic testing: Helpful in hereditary conditions such as ATTR
- Blood and urine tests: Identify abnormal light chains or amyloid proteins
8. Treatment Approaches Targeting Misfolding
Therapies for amyloidosis seek to decrease the formation of the misfolded protein, inhibit aggregation, or enhance clearance from tissues.
Current Therapies:
- AL Amyloidosis: Chemotherapy, stem cell transplant
- ATTR Amyloidosis: Tafamidis, Patisiran, Inotersen
- AA Amyloidosis: Management of underlying inflammation
- Organ transplant: In severe cases
New Therapies:
- Chaperone therapy: Facilitates proper folding of proteins
- Immunotherapy: Aims for removal of amyloid deposits
- RNA interference: Silences genes that create amyloidogenic proteins
9. Research and Future Directions
Scientists are working on new methods to treat and understand protein misfolding disorders, such as:
- AI-based protein folding models (e.g., AlphaFold)
- Early detection biomarkers
- Vaccine development for amyloid diseases
- Gene editing tools (e.g., CRISPR)
These developments hold promise for earlier diagnosis, improved treatment, and even eventual prevention.
10. Coping with a Protein Misfolding Disorder
For caregivers and patients, recognizing amyloidosis as a misfolding disorder can:
- Increase knowledge of the disease
- Foster early medical intervention
- Facilitate clinical trial participation
- Decrease stigma of intricate symptoms
Coping Strategies:
- Connect with patient groups such as AmyloidosisSupport
- Take regular follow-ups
- Lead a healthy lifestyle to facilitate organ function
- Stay updated on treatment alternatives
Frequently Asked Questions (FAQs)
Q1: Why does a protein misfold?
Proper folding can be disrupted by genetic mutations, environmental stress, aging, or cell damage.
Q2: Are all protein misfolding disorders identical?
No, there are various disorders with various proteins and symptoms but the same general mechanism of misfolding.
Q3: Is it possible to reverse protein misfolding?
There are some therapies that attempt to refold proteins properly or inhibit misfolding, but all of them are not reversible.
Q4: Is amyloidosis common?
It’s a rare condition but usually underdiagnosed because it presents with nonspecific symptoms.
Q5: Can amyloidosis be prevented?
Heredity forms might not be preventable, but proper control of chronic diseases can lower the risk of developing certain types such as AA amyloidosis.
Amyloidosis is a prototypic protein misfolding disorder, with the misfolding of certain proteins resulting in toxic aggregation and organ injury. Understanding this fundamental mechanism is important for diagnosis, treatment, and investigation. As the science unfolds the secrets of protein folding, new therapies are in development that hold out promise for patients with amyloidosis.
At AmyloidosisSupport, we are committed to educating, empowering, and supporting amyloidosis patients and those affected by amyloidosis. Learning about the nature of protein misfolding brings us nearer to more effective treatments and a better quality of life.

