Amyloid Deposits Explained
Table of Contents
What Are Amyloid Deposits?
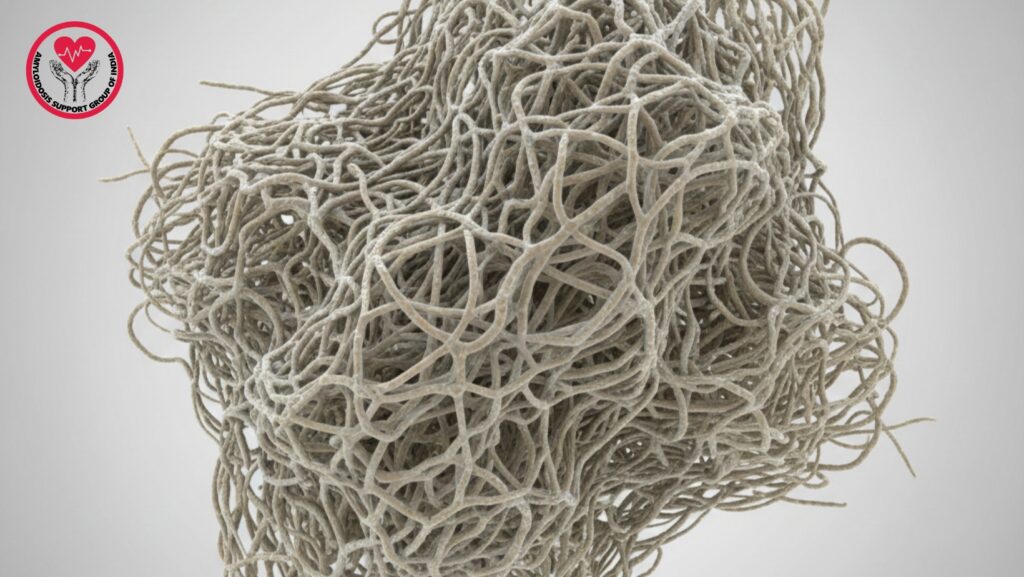
Amyloid deposits are abnormal protein clumps that build up in various organs, including the heart, kidneys, liver, nerves, and soft tissues. These deposits are not normal. They form when certain proteins misfold, lose their natural shape, and group together into long, rigid structures called amyloid fibrils.
These fibrils then accumulate into larger masses, disrupting the normal function of tissues and causing symptoms based on where they form.
Why Are Amyloid Deposits Mostly Fibrils (90%)?
Research indicates that about 90% of an amyloid deposit consists of fibrils because the fibril form is the most stable, compact, and rigid structure that misfolded proteins can take. When proteins misfold, they quickly stack into beta-sheet-rich fibrils, which become the main component of the deposit.
The remaining 10% typically includes:
- Serum amyloid P component (SAP)
- Glycosaminoglycans (GAGs)
- Lipids
- Other trapped proteins from the tissue microenvironment
Together, these additional components act like “cement,” helping to stabilize the fibril mass.
Understanding Amyloid Fibrils
How Fibrils Form
Amyloid fibrils form in three main stages:
Nucleation
Proteins start to misfold due to genetic mutations, chronic inflammation, plasma cell disorders, or metabolic factors.
Elongation
Misfolded proteins attach to the nucleus, quickly forming long, thread-like fibrils.
Aggregation
These fibrils gather together, creating visible amyloid deposits.
This is why fibrils dominate the deposit—they keep growing and attract more proteins into the fibril structure.
Types of Amyloid That Form Fibrils
Not all proteins turn into amyloid. Only specific unstable or abnormal proteins misfold into fibrils.
Common types include:
- AL (Light-chain Amyloidosis) – caused by plasma cells
- AA Amyloidosis – due to long-term inflammation
- ATTR (Transthyretin Amyloidosis) – hereditary or age-related
- Aβ Amyloid – found in Alzheimer’s disease
- ALECT2 – common in liver and kidney amyloid
Each type forms fibrils with slightly different properties but maintains the same beta-sheet-rich structure.
Why Fibrils Are So Hard for the Body to Remove
Highly Stable Structure
Amyloid fibrils resist:
- Enzymatic breakdown
- Chemical degradation
- Heat and pH changes
This stability allows them to remain in tissues for years.
Cross-Beta Structure
The cross-beta sheet structure provides:
- Rigidity
- Insolubility
- Tensile strength similar to steel (per nanometer scale)
This is the main reason fibrils build up faster than the body can clear them.
How Fibrils Damage Organs
Mechanical Pressure
Deposits push against normal cells, reducing blood flow and harming tissue structure.
Toxicity of Misfolded Proteins
Before becoming fibrils, the intermediate misfolded proteins (oligomers) can be very toxic to cells.
Stiffening of Tissues
Fibril-heavy deposits make organs stiff:
- Restrictive cardiomyopathy in the heart
- Enlarged liver
- Nerve conduction issues
- Kidney filtration problems
Barrier to Cellular Repair
The 90% fibril density obstructs healing and regeneration.
Is the 90% Fibril Ratio the Same in All Types of Amyloidosis?
Not exactly—but it’s similar. Most amyloid deposits in medical studies range from 85 to 95% fibrils, depending on:
- Protein type
- Organ involved
- Age of the deposit
- Co-deposited molecules
ATTR and AL typically show the highest concentration of fibrils.
Diagnosing Amyloid Fibrils
Biopsy (Gold Standard)
A sample from the abdominal fat pad, kidney, heart, or bone marrow reveals:
- Congo red positivity
- Apple-green birefringence under polarizing light
- Electron microscopy showing long fibrils
Mass Spectrometry
Identifies the specific type of amyloid protein.
Imaging
- Echocardiography
- Cardiac MRI
- Nuclear scans for ATTR
These methods show stiffness and organ involvement caused by fibrils.
Can Amyloid Fibrils Be Treated or Removed?
Treatment varies by type:
AL Amyloidosis
- Chemotherapy
- Stem cell transplantation
- Plasma cell-targeted drugs
ATTR Amyloidosis
- Tafamidis
- Patisiran / Vutrisiran
- Liver transplant (hereditary forms)
AA Amyloidosis
- Treating the source of inflammation
Experimental Research
Scientists are exploring ways to:
- Dissolve fibrils
- Block fibril formation
- Improve macrophage clearance
- Stabilize precursor proteins
Future treatments aim to directly remove the fibril mass from organs.
Simple Summary
Amyloid deposits are dangerous because 90% of their structure consists of dense, stable amyloid fibrils. These fibrils are very difficult for the body to break down, leading to organ damage over time. Understanding how fibrils form helps with early diagnosis and targeted treatment.