Table of Contents
Introduction-amyloid fibrils toxic
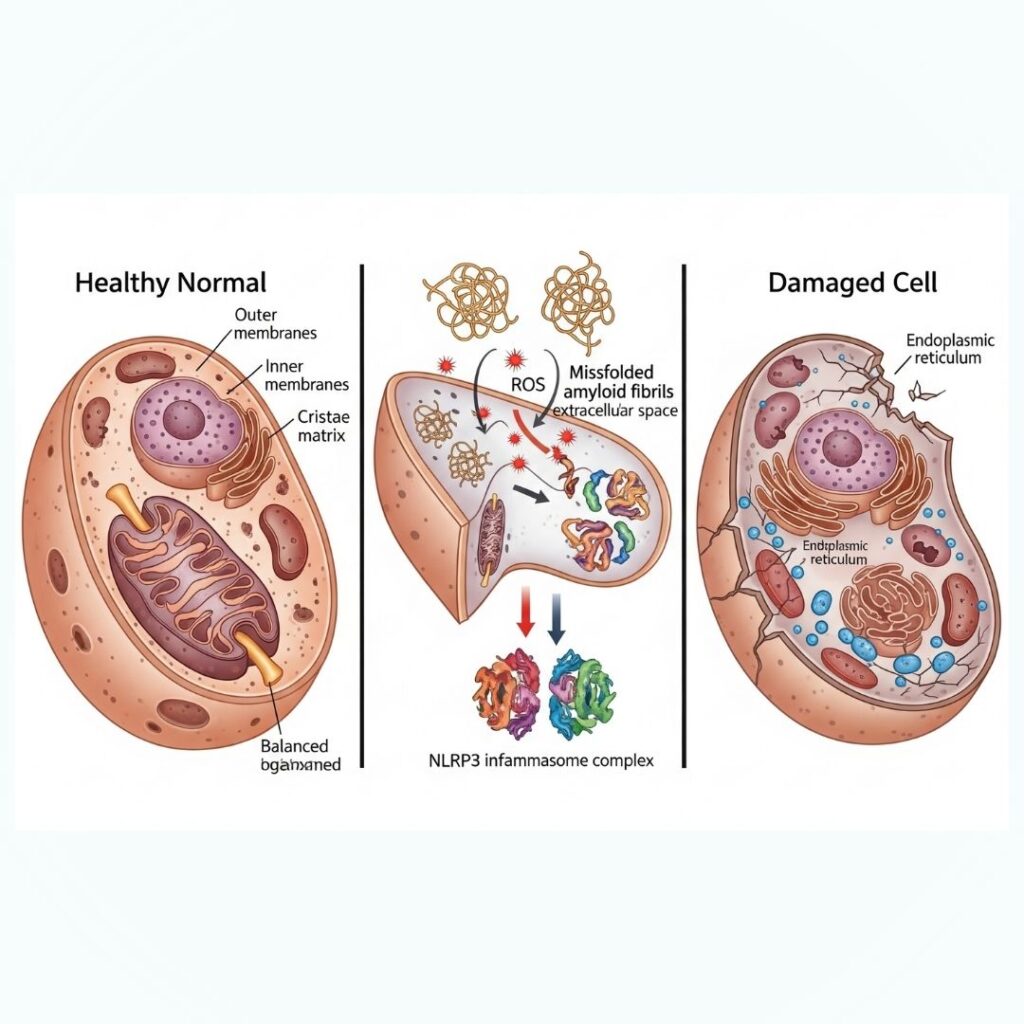
Amyloidosis is a multifaceted disease characterized by the deposition of amyloid fibrils in organs and tissues, culminating in progressive damage and dysfunction. Amyloid fibrils are insoluble protein aggregates that are refractory to normal cell mechanisms of degradation. Although the accumulation is toxic, their toxicity goes much further than their mere presence. They inflict cytotoxicity by a variety of mechanisms including the production of reactive oxygen species (ROS), activation of inflammatory processes (particularly the NLRP3 inflammasome), and direct membrane disruption. This paper examines these mechanisms in detail to gain better insight into the molecular basis of amyloid-related organ toxicity.
Section 1: What Are Amyloid Fibrils?
Amyloid fibrils result from misfolded proteins taking on a beta-sheet dominant conformation and aggregating to form insoluble, fibrillar structures. Fibrils are described by their stability, resistance to proteolysis, and capacity for binding certain dyes such as Congo red with the appearance of apple-green birefringence under polarized light.
Key Features:
- Made up of stacked beta-sheets
- Derived from misfolded precursor proteins
- Have fibrillar structure under electron microscopy
- Linked to more than 30 known proteins (e.g., AL, AA, ATTR)
Section 2: Protein Misfolding and Aggregation Mechanism
In normal circumstances, proteins fold into specific three-dimensional structures to perform optimally. But genetic mutations, environmental stressors, oxidative damage, and aging can disrupt the process. Misfolded proteins reveal hydrophobic residues, which expose them to aggregation.
Phases:
- Native protein destabilization
- Abnormal beta-sheet folding
- Formation of oligomers
- Fibril formation and tissue deposition
Section 3: Reactive Oxygen Species (ROS) Generation
One of the major toxicities of amyloid fibrils is their capacity to generate ROS. These reactive species induce oxidative stress, damaging cellular structures like lipids, proteins, and DNA.
Implications of ROS in Amyloidosis:
- Cellular membrane lipid peroxidation
- Mitochondrial impairment
- DNA fragmentation
- Induction of apoptotic pathways
Research indicates that cells treated with amyloid fibrils have high levels of ROS, activating a chain reaction of oxidative damage and cell death.
Section 4: Inflammasome Activation (NLRP3)
Amyloid fibrils are able to activate the innate immune response by stimulating the NLRP3 inflammasome, a protein complex that recognizes pathogenic stimuli.
Mechanism:
- Amyloid fibrils as danger-associated molecular patterns (DAMPs)
- Bind pattern recognition receptors (PRRs)
- Induce assembly of NLRP3 inflammasome
- Activation of caspase-1 results in release of IL-1β and IL-18
- Facilitates chronic inflammation and tissue injury
Activation of inflammasomes is specifically pertinent in diseases such as Alzheimer’s, AL amyloidosis systemic, and AA amyloidosis.
Section 5: Cellular Membrane Disruption
Amyloid fibrils are also cytotoxic directly by incorporating into lipid bilayers, disrupting cellular membrane integrity.
Major Effects:
- Leakage of the membrane and ion imbalance
- Calcium influx through pore formation
- Mitochondrial membrane potential loss
- Initiation of necrosis or apoptosis
This process is well described in neuronal amyloidoses, in which synaptic dysfunction and cell death are conspicuous.
Section 6: Amyloid Toxicity in Specific Organs
Amyloid fibrils have tissue-specific toxicities based on amyloid type and organ of deposition.
- Heart (ATTR, AL):
- Myocardial stiffening
- Heart failure and arrhythmias
- Kidneys (AA, AL):
- Proteinuria
- Renal failure due to progressive nature
- Nervous System (ATTR, Aβ):
- Peripheral neuropathy
- Cognitive impairment (e.g., Alzheimer’s disease)
- Liver and Spleen:
- Hepatomegaly
- Splenomegaly
Section 7: Current Research and Therapeutic Approaches
The realization of amyloid fibril toxicity has spurred novel therapeutic targets for the purpose of blocking aggregation, neutralizing fibrils, or facilitating their removal.
Pruising Approaches:
- TTR stabilizers (e.g., tafamidis)
- Fibril disruptors (e.g., doxycycline, EGCG)
- Immunotherapy (monoclonal antibodies)
- NLRP3 small molecule inhibitors
Studies are ongoing to come up with safer and more effective measures to reduce the damage from amyloid deposition.
Conclusion
Amyloid fibrils are not static protein deposits but dynamic drivers of cellular dysfunction and tissue injury. Their toxicity stems from several synergistic mechanisms: oxidative stress, inflammation through inflammasome activation, and physical disruption of cell structures. A more profound knowledge of these pathways is necessary to develop targeted therapies to better fight amyloidosis.
By revealing the poisonous nature of amyloid fibrils, this article hopes to empower patients, caregivers, and researchers as they battle this debilitating disease.

