Table of Contents
Introduction
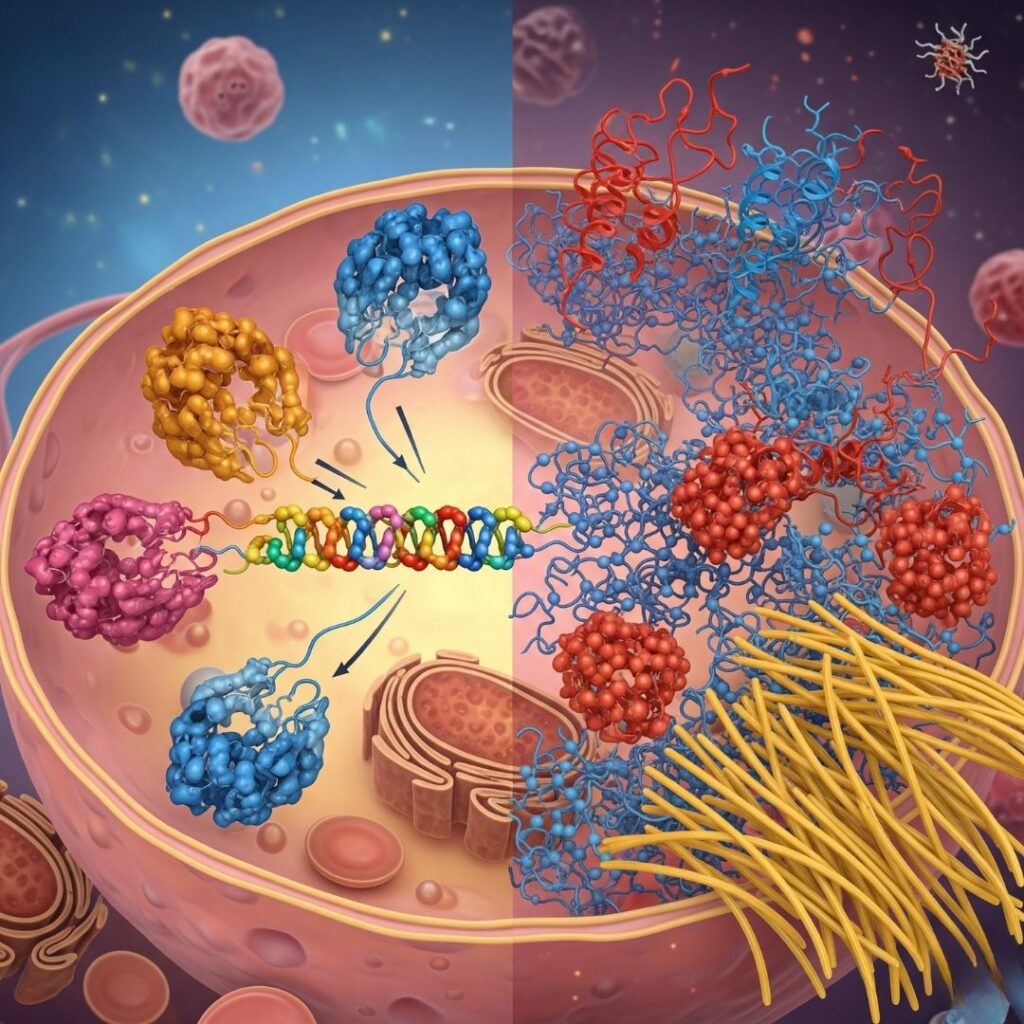
Proteins are the workhorses of the human body, involved in virtually every biological process. To function properly, proteins must fold into precise three-dimensional shapes. This folding process is complex and prone to error. Molecular chaperones are essential players in guiding proteins to fold correctly and preventing the accumulation of misfolded proteins. When chaperone systems fail, the risk of protein misfolding and aggregation increases, contributing to diseases such as amyloidosis.
This article explores the critical role of chaperones in protein folding, their mechanisms of action, and how their dysfunction contributes to protein aggregation and amyloid-related diseases.
1. Understanding Protein Folding
Proteins begin as linear chains of amino acids synthesized by ribosomes. For them to perform specific biological functions, these chains must fold into precise three-dimensional structures. The process is governed by:
- Hydrophobic and hydrophilic interactions
- Hydrogen bonds
- Electrostatic interactions
- Van der Waals forces
But due to the intracellular crowding and kinetic heterogeneity, proteins usually need help to get properly folded.
2. Introduction to Molecular Chaperones
Molecular chaperones are a heterogeneous set of proteins that aid the folding of newly synthesized polypeptides and the refolding of misfolded or aggregated proteins. They do not become part of the mature protein structure but are involved in preventing inappropriate interactions and aggregation.
Chaperones may be grouped into a number of families according to structure and function:
- Hsp70 family
- Hsp90 family
- Chaperonins (e.g., GroEL/GroES in bacteria)
- Small heat shock proteins (sHSPs)
3. Mechanisms of Action
Chaperones act in different ways to help protein folding:
- Stabilizing unfolded proteins: Prevent aggregation by protecting hydrophobic areas.
- ATP-driven conformational cycles: Employ energy from ATP hydrolysis to bind and release substrates, enabling proper folding.
- Providing isolated folding environments: Chaperonins form enclosed chambers where a single protein can fold without interference.
These processes are tightly regulated and essential for cellular homeostasis.
4. Chaperones and Protein Misfolding
When chaperones are overwhelmed, mutated, or otherwise impaired, the balance shifts toward misfolding. Misfolded proteins can aggregate into toxic oligomers or insoluble fibrils, contributing to various disorders:
- Neurodegenerative diseases: Alzheimer’s, Parkinson’s, Huntington’s
- Systemic amyloidosis: Including AL, AA, and ATTR types
In amyloidosis, misfolded proteins rich in β-sheet structure aggregate into amyloid fibrils, which deposit in organs and impair function.
5. Chaperones in Amyloidosis
In amyloidosis, chaperones fail to refold or degrade misfolded proteins, allowing them to aggregate. Some key observations include:
- Heat shock response impairment: Reduces chaperone expression
- Oxidative stress and aging: Affect chaperone function
- Mutations in chaperone genes: Can reduce efficiency
Chaperones also influence the balance between protective and pathogenic folding pathways. For instance:
- Clusterin and Hsp70: Can bind amyloidogenic intermediates and inhibit aggregation
- Serum amyloid P component (SAP): Binds to amyloid fibrils and stabilizes them, paradoxically promoting deposition
6. Therapeutic Implications
Targeting chaperone pathways offers therapeutic potential:
- Upregulating chaperones: Using heat shock protein inducers
- Chemical chaperones: Small molecules that mimic chaperone activity
- Gene therapy: Enhancing expression of beneficial chaperones
- Inhibiting SAP: To destabilize existing amyloid deposits
Research is ongoing into drugs that can boost natural chaperone systems or artificially restore folding capacity.
7. Environmental and Genetic Factors Affecting Chaperones
Factors that impair chaperone function include:
- Aging: Natural decline in cellular stress responses
- Genetic mutations: Affect expression or function of chaperones
- Inflammation and stress: Increase misfolded protein burden
- Toxic metabolites: From conditions like diabetes or kidney disease
Understanding these risk factors may help prevent or mitigate amyloidosis progression.
8. Future Directions in Research
Emerging research is exploring:
- Chaperone network modulation: Enhancing collaboration among different chaperones
- Personalized medicine: Tailoring therapy based on chaperone gene variants
- Biomarker development: Using chaperone levels as indicators of disease progression or treatment response
Conclusion
Molecular chaperones play a fundamental role in maintaining protein homeostasis. Their dysfunction contributes to the formation of misfolded protein aggregates, including the amyloid fibrils seen in various forms of amyloidosis. A deeper understanding of chaperone mechanisms and how to manipulate them therapeutically may pave the way for novel treatments and improved outcomes for patients facing protein misfolding disorders.

