Understanding the Gamma-Globulin Area in Electrophoresis: Immunoglobulin Movement and Clinical Relevance
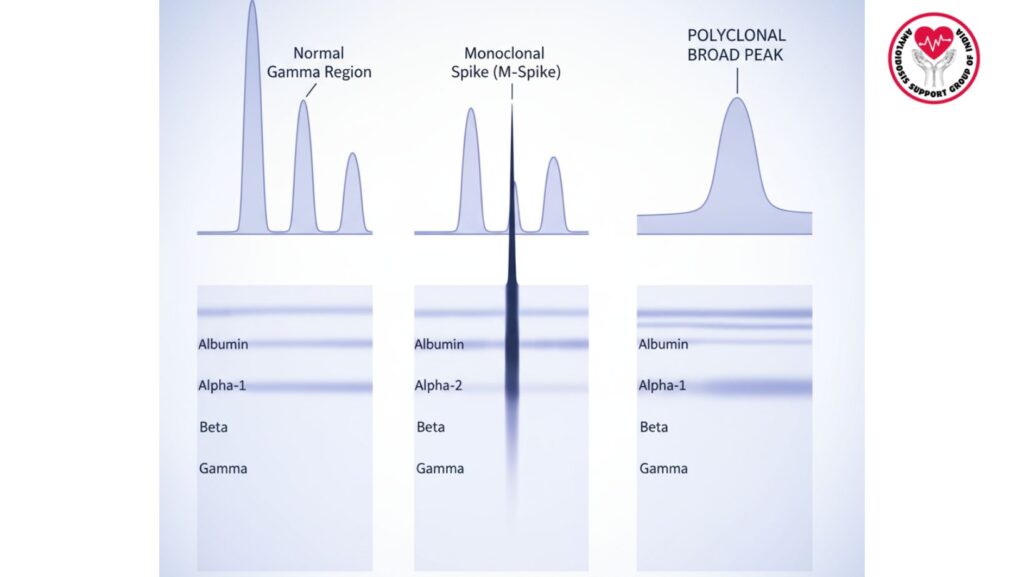
Table of Contents
Introduction
Serum protein electrophoresis (SPE) is an important laboratory method to distinguish serum proteins according to charge, size, and mobility under an electric current. The gamma-globulin area, one of the most clinically important areas identified during SPE, is composed mainly of immunoglobulins (antibodies).
It is essential to understand the gamma-globulin area for:
- Detection of monoclonal gammopathies like multiple myeloma and AL amyloidosis
- Recognition of polyclonal hypergammaglobulinemia caused by infections, autoimmune diseases, or chronic inflammation
- Following therapy response in plasma cell disorders
This article gives a complete picture of: - The composition and structure of the gamma-globulin region
- Immunoglobulins during electrophoresis
- Interpretation of SPE patterns
- Clinical significance in diagnosing plasma cell and immune disorders
- Combination with other laboratory methodologies
Fundamentals of Serum Protein Electrophoresis
Fractions of Protein in SPE
Serum proteins are divided into five major fractions:
- Albumin – Most common, migrates fastest towards the anode
- Alpha-1 globulins – Contain alpha-1 antitrypsin and alpha-1 acid glycoprotein
- Alpha-2 globulins – Contain haptoglobin, ceruloplasmin, and macroglobulin
- Beta globulins – Contain transferrin, complement proteins, and certain immunoglobulins
- Gamma-globulins – Mainly immunoglobulins (IgG, IgA, IgM, IgE, IgD)
Electrophoretic Principles
- Proteins move according to net charge and size
- Albumin, with extreme negative charge, moves most rapidly to the anode (positive electrode)
- Gamma-globulins, with moderate negative charge, move less quickly, creating a clear gamma region
What is the Gamma-Globulin Region?
Definition
The gamma-globulin region is the region of the electrophoretic gel where immunoglobulins concentrate during SPE. It is nearest the cathode relative to albumin and alpha/beta globulins.
Composition
- Composed mainly of IgG, the most common antibody found in serum
- Contains IgA and IgM, adding to the width and form of the gamma region
- Small contributions from IgD and IgE
Visualization
- Staining of the gel (e.g., Coomassie Brilliant Blue) shows a band or broad peak in the gamma region
- Monoclonal proteins show as sharp, narrow spikes (M-protein)
- Polyclonal increases yield broad, diffuse elevations
Factors Affecting Migration of Gamma-Globulin
- Net Charge of Immunoglobulins
- Controls speed and direction within the electric field
- Size and Molecular Weight
- More massive antibodies (IgM pentamers) will have slower migration than lighter IgG monomers
- Gel Concentration and Composition
- Higher concentration gels can slow migration, impacting peak resolution
- Buffer pH
- Permits charge state of immunoglobulins for reproducible migration
- Temperature and Voltage
- Overheating or high voltage distorts gamma region patterns
Clinical Significance
Monoclonal Gammopathies
- Multiple Myeloma
- M-protein creates a narrow spike in the gamma region
- Size of peak is related to the burden of disease
- AL Amyloidosis
- Monoclonal light chains can cause spikes in the gamma region
- Waldenström Macroglobulinemia
- IgM monoclonal proteins are seen as narrow or broad gamma peaks
Polyclonal Hypergammaglobulinemia
- General rise in gamma region due to immune activation
- Etiologies include:
- Chronic infections (hepatitis, HIV)
- Autoimmune illnesses (SLE, rheumatoid arthritis)
- Chronic liver disease
Hypogammaglobulinemia
- Decreased height of gamma region points towards low immunoglobulin levels
- Observed in:
- Primary immunodeficiencies
- Immunosuppression due to chemotherapy
- End-stage renal or liver disease
Interpreting SPE Patterns
| Pattern | Description | Clinical Implications |
| ——————————————————————- | ————————- | —————————— |
| Sharp narrow spike | Monoclonal immunoglobulin | Multiple myeloma, AL amyloidosis, MGUS |
| Wide gamma rise | Polyclonal rise | Infection, autoimmune disorder, chronic inflammation |
| Flattened or decreased gamma | Hypogammaglobulinemia | Immunodeficiency, post-transplant, chemotherapy |
| Biclonal peaks | Two separate spikes | Two clone myeloma with multiple myeloma, therapeutic antibody interference |
Integration with Immunofixation Electrophoresis (IFE)
- IFE verifies type of immunoglobulin (IgG, IgA, IgM, kappa/lambda light chains)
- Discriminates monoclonal vs polyclonal bands
- Critical for accurate diagnosis and monitoring
Gamma-Globulin Region in Monitoring Disease
Therapy Response
- M-protein spike decrease is a sign of successful therapy
- Recurrent or rising spike points to residual disease or relapse
Stem Cell Transplantation
- SPE gamma region measurement is used to monitor immune recovery after transplant
- Can identify return of monoclonal proteins
Newer Methods
- Mass spectrometry (iMS-LC) improves gamma-region analysis
- MRD monitoring combines gamma region information with molecular assays
Frequent Errors and Artifacts
Overlapping beta-globulin peaks can hide gamma region
- Therapeutic monoclonal antibodies can form artificial spikes
- Sample hemolysis or lipemia can distort band patterns
- Proper buffer and gel preparation ensures proper visualization
FAQs
Q1: Why is the gamma-globulin region significant?
It is representative of immunoglobulins that are essential for identifying monoclonal or polyclonal gammopathies.
Q2: Can all immunoglobulins be identified in SPE?
Mainly IgG, IgA, and IgM. IgD and IgE are in very low concentrations.
Q3: What does a sharp gamma spike suggest?
A monoclonal protein (M-protein), commonly found in multiple myeloma or AL amyloidosis.
Q4: How does polyclonal hypergammaglobulinemia manifest?
As a general, diffuse rise of the gamma region, not a definate spike.
Q5: Do drugs influence the gamma region?
Yes, therapeutic monoclonal antibodies can produce artifacts that are spike-like.
Conclusion
The gamma-globulin region is an important area in serum protein electrophoresis reflecting immunoglobulin migration. Knowledge of its patterns enables clinicians to:
- Identify monoclonal and polyclonal gammopathies
- Evaluate immunity function and response to therapy
- Monitor for remnant disease or recurrence in plasma cell diseases
Through the integration of SPE and immunofixation with advanced mass spectrometry methods, precise identification and measurement of immunoglobulins in the gamma region has become a vital asset in diagnostics in the clinical setting, hematology, and immunology research.

