Gastrointestinal Symptoms of Systemic Amyloidosis: Identification and Management
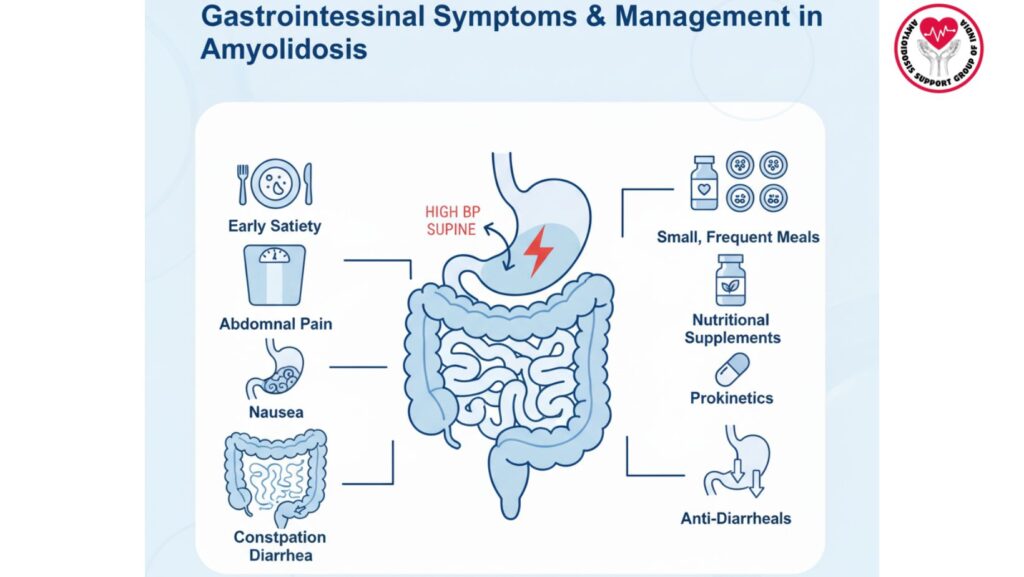
Table of Contents
Introduction
Gastrointestinal (GI) involvement is a frequent and clinically relevant manifestation of systemic amyloidosis, especially in AL (light-chain) and ATTR (transthyretin) amyloidosis. Amyloid deposits within the GI tract, autonomic nervous system, and vasculature impair regular digestive physiology, and cause a broad spectrum of symptoms.
Patients can present with early satiety, weight loss, abdominal pain, nausea, constipation, or diarrhea, frequently impacting nutrition, quality of life, and global prognosis. Early detection of GI involvement is important for prompt treatment.
This review article gives a thorough overview on pathophysiology, clinical presentation, diagnostic approach, and management of GI symptoms in systemic amyloidosis.
1. Pathophysiology of GI Involvement
1.1 Amyloid Deposition
- Amyloid protein deposition in submucosa, muscularis propria, and autonomic nerves of the GI tract.
- Results in motility disorders, impaired absorption, and vascular fragility.
1.2 Autonomic Neuropathy
- Abnormality in enteric nervous system signaling leads to dysmotility, delayed gastric emptying, and constipation.
- May lead to diarrhea due to bacterial overgrowth and loss of sphincter control.
1.3 Vascular Involvement
- Weak blood vessels can lead to microbleeds, ischemia, and abdominal pain.
- Causes malabsorption and loss of protein in extreme cases.
2. Common Gastrointestinal Symptoms
2.1 Early Satiety
- Fullness after eating small amounts
- Usually secondary to gastroparesis or decreased gastric compliance
2.2 Weight Loss
- Due to decreased intake, malabsorption, or chronic nausea
- May lead to cachexia in advanced amyloidosis
2.3 Abdominal Pain
- Crampy or diffuse
- Associated with motility disorders, ischemia, or amyloid infiltration
2.4 Nausea and Vomiting
- Often linked to delayed gastric emptying
- May result in electrolyte imbalance and dehydration
2.5 Constipation
- Self-treatment due to colonic dysmotility
- Worsened by autonomic neuropathy and medications
2.6 Diarrhea
- Oftentimes intermittent
- May result from malabsorption, small intestinal bacterial overgrowth (SIBO), or protein-losing enteropathy
3. Clinical Evaluation
3.1 History
- Evaluate symptom initiation, severity, and precipitants
- Dietary patterns, frequency of bowel movements, and weight changes
- Associated autonomic symptoms: OH, urinary dysfunction
3.2 Physical Exam
- Abdominal bloating, tenderness, or organomegaly
- Malnutrition signs (muscle wasting, low BMI)
- Peripheral neuropathy evidence
3.3 Lab Tests
- CBC, electrolytes, liver function tests, renal function tests
- Serum albumin and prealbumin for assessment of nutrition
- Infection, fat malabsorption, or protein loss in stool studies
3.4 Imaging and Endoscopy
- Abdominal ultrasound or CT for structural pathology
- Endoscopy with biopsy to establish amyloid deposition
- Gastric emptying studies for gastroparesis
4. Management Strategies
4.1 Management and Lifestyle Measures
- Frequent small meals for early satiety
- High-calorie, nutrient-dense diets
- Sufficient hydration
- Avoiding irritating foods (e.g., lactose)
4.2 Drug Interventions
4.2.1 Gastroparesis
- Prokinetic drugs: Metoclopramide, domperidone, or erythromycin
- Enhances gastric emptying and alleviates nausea
4.2.2 Diarrhea
- Antimotility drugs: Loperamide, diphenoxylate-atropine
- Treat the underlying cause such as SIBO with antibiotics
4.2.3 Constipation
- Osmotic or stimulant laxatives (e.g., polyethylene glycol, senna)
- Induce regular bowel movements and minimize abdominal pain
4.2.4 Nausea and Vomiting
- Anti-emetics: Ondansetron, metoclopramide
- Treat underlying delayed gastric emptying
4.2.5 Malabsorption and Nutritional Support
- Oral nutritional supplements
- Enteral feeding if intake is poor
- Watch for vitamin and mineral deficiency
4.3 Advanced Interventions
4.3.1 Endoscopic or Surgical Management
- Only rarely needed for severe obstruction or bleeding
- Diagnosis is confirmed and therapy directed by biopsies
4.3.2 Autonomic Modulation
- Treat underlying autonomic dysfunction that causes motility disorders
5. Monitoring and Follow-Up
- Monitor weight, nutritional status, and GI symptoms
- Periodic laboratory tests: albumin, electrolytes, vitamins
- Modify therapy according to symptom control and tolerance
- Multidisciplinary management: gastroenterology, nutrition, neurology
6. Case Examples
Case 1: Gastroparesis in AL Amyloidosis
- 65-year-old with early satiety and weight loss
- Intervention: Small meals, metoclopramide 10 mg TID
- Outcome: Improved gastric emptying, weight stabilization
Case 2: Diarrhea in ATTR Amyloidosis
- Intermittent diarrhea in a 60-year-old with malnutrition
- Intervention: Loperamide, rifaximin in SIBO, dietary modification
- Outcome: Decreased diarrhea, increased hydration, weight stabilization
7. Special Considerations in Amyloidosi
- Cardiac involvement can restrict fluid management
- Renal amyloidosis can worsen electrolyte imbalance
- Polypharmacy can exacerbate constipation or diarrhea
- Individualized care is essential for symptom control and nutrition
8. Emerging Therapies and Research
- Targeted amyloid therapies (e.g., tafamidis, patisiran) can indirectly reduce GI symptoms
- Modulation of gut microbiome for SIBO and diarrhea
- Prokinetic agents being studied
- Wearable devices for GI motility monitoring and early symptom detection
9. Patient Education
- Identify and report early satiety, weight loss, or bowel habit changes
- Continue to keep food and symptom diary
- Adhere to dietary and fluid guidelines
- Know medication aims and potential side effects
- Involve nutritionists and gastroenterologists in long-term care
10. Conclusion
Gastrointestinal involvement in systemic amyloidosis is prevalent, multifactorial, and has a profound effect on nutrition, quality of life, and disease outcomes.
Key points:
- Early detection of symptoms such as early satiety, weight loss, abdominal pain, nausea, constipation, and diarrhea is vital
- Management is through dietary changes, pharmacologic treatment, and nutritional support
- Monitoring and multidisciplinary management enhance quality of life and prevent complications
- New therapies aimed at preventing amyloid deposition can provide symptom relief in the future
Early intervention, tailored care, and patient education are imperative to maximizing GI function and general health in systemic amyloidosis patients.

