Table of Contents
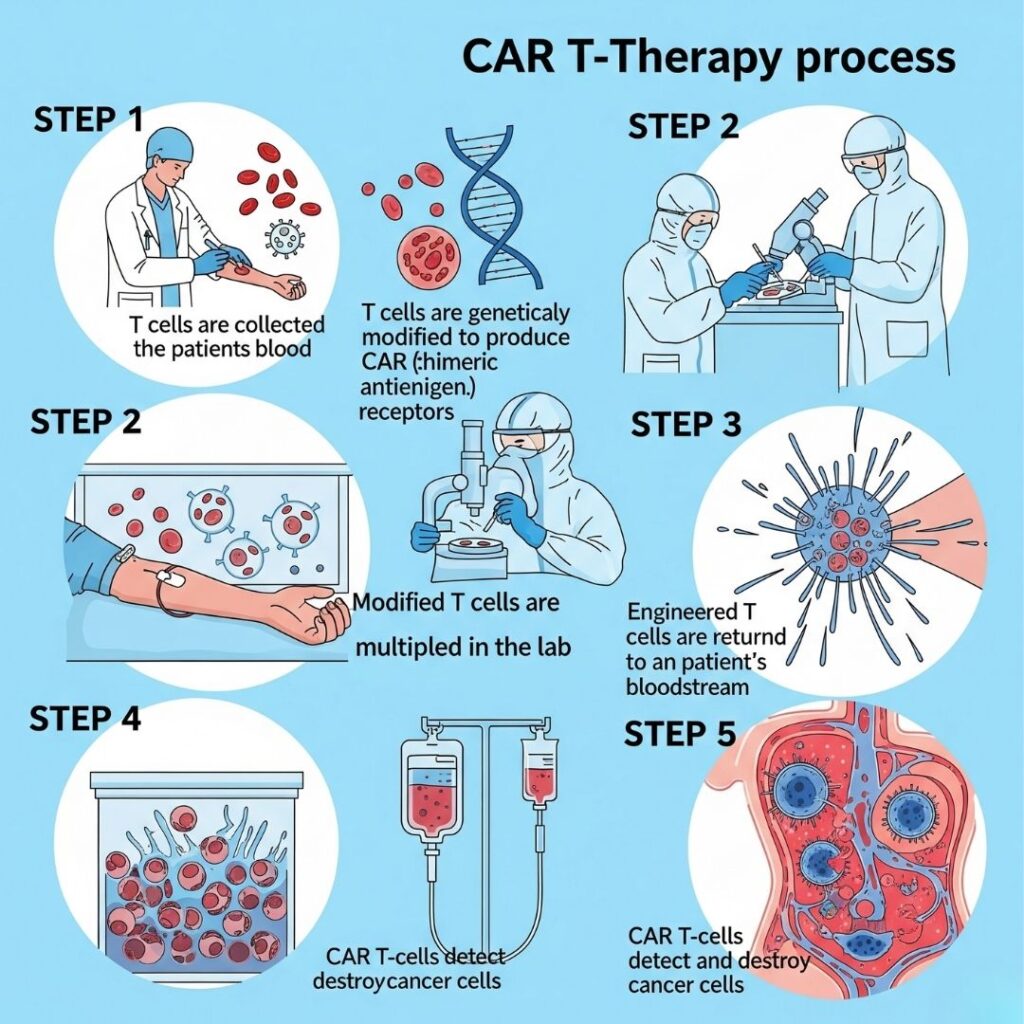
CAR T-cell therapy Work(Chimeric Antigen Receptor T-cell therapy) is one of the most cutting-edge and tailored therapies for blood cancers such as leukemia, lymphoma, and multiple myeloma. It involves a patient’s own immune cells, which are genetically modified to identify and destroy cancer cells.
Overview: The Main Idea
CAR T-cell therapy reprograms the patient’s T-cells (white blood cells) to identify specific markers (antigens) on the outside of cancer cells. The reprogrammed T-cells are then reinfused into the patient’s body, where they search out and kill the cancer with great accuracy.
Step-by-Step Breakdown: How CAR T-Cell Therapy Works
1. T-Cell Collection (Leukapheresis)
- The procedure begins with a blood draw from the patient.
- With the aid of a machine, T-cells are isolated from the other blood components.
- The other blood is returned to the patient.
- The process normally takes 2–3 hours.
2. Genetic Modification in the Laboratory
- The T-cells collected are transported to a specialized laboratory.
- Scientists employ an harmless virus to deliver a gene that produces the Chimeric Antigen Receptor (CAR) into the T-cells.
- These CARs are proteins that assist the T-cells to recognize and attach to special antigens present on cancer cells (e.g., CD19 on B-cell leukemias and lymphomas).
3. Cell Expansion (Growing the Army)
- After modification, the CAR T-cells are permitted to grow in huge numbers.
- Billions of CAR T-cells are produced at this stage.
- This process lasts for 2 to 3 weeks.
4. Conditioning Chemotherapy (Lymphodepletion)
- The patient gets a short course of chemotherapy before the infusion.
- This process eliminates room in the immune system and depletes other immune cells that could get in the way of the CAR T-cells.
5. CAR T-Cell Infusion
- The CAR T-cells are now administered back into the patient’s blood circulation through IV.
- This is a single, one-time dose, which is sometimes administered in a hospital.
6. Cancer Cell Recognition and Attack
- The reprogrammed CAR T-cells tour the bloodstream.
- Upon encountering cancer cells bearing the target antigen (such as CD19), the CAR T-cells attach to them.
- The attachment stimulates the T-cells to destroy the cancer cells via:
- Perforins (puncture holes in cancer cells)
- Granzymes (cause programmed cell death)
- Cytokine release (to summon additional immune cells)
7. T-Cell Persistence and Memory
- CAR T-cells can last in the body for months or even years, ongoing to watch over and attack any persistent or recurring cancer cells.
What Sets CAR T-Cell Therapy Apart?
In contrast to conventional therapies (such as chemo or radiation), CAR T-cell therapy is:
- Personalized – Designed to the patient’s immune system
- Targeted – Attacking only cells bearing the particular cancer antigen
- Potentially Curative – Even in patients who had failed earlier treatments
- Long-term – Certain CAR T-cells remain in the body and function as “memory T-cells”
What Antigens Does CAR T-Cell Therapy Target?
Most of today’s FDA-approved CAR T-cell treatments target:
- CD19 – Expressed on B-cells (for treating B-cell leukemias and lymphomas)
- BCMA – Expressed on cancerous plasma cells (for multiple myeloma)
Investigations are extended to include: - HER2, EGFR, mesothelin – Targets for solid tumors
- Dual CARs – T-cells that are designed to recognize two antigens at the same time
How Long Does the Whole Process Take?
- It can take 3–4 weeks from T-cell collection to infusion
- Monitoring after infusion takes 2 to 3 weeks, usually in hospital
- Recovery and immune monitoring can persist for several months
Side Effects and Monitoring
CAR T-cell treatment can have serious side effects, which is why it’s administered in specialized units.
1. Cytokine Release Syndrome (CRS)
- Overreaction of immune system
- Side effects: fever, low blood pressure, trouble breathing
- Treated with medications such as tocilizumab
2. Neurotoxicity (ICANS)
- Side effects: confusion, trouble speaking, tremors, seizures
- Typically reversible with steroids and supportive treatment
3. Low Blood Counts and Infections
- Caused by lymphodepleting chemo or immunosuppression
- Patients might require growth factors, antibiotics, or IVIG
Success Rates and Outcomes
CAR T-cell therapy has truly impressive outcomes, particularly for blood cancers:
- Childhood and young adult B-cell ALL: age-adjusted remission rates of 80–90%
- Large B-cell lymphoma: 50–70% response rates
- Multiple myeloma (with BCMA CAR T): encouraging outcomes in relapsed/refractory patients
Remission duration is variable; some have sustained remission, while others have relapsed and need additional treatment.
Who Is Eligible for CAR T-Cell Therapy?
Generally for patients who
- Have relapsed or refractory blood cancers
- Have attempted and failed standard therapies
- Are in good enough health to tolerate treatment
- Have a target antigen (such as CD19 or BCMA) expressed on their cancer cells
A full assessment is performed by the treatment team to decide if the patient is eligible.
Future of CAR T-Cell Therapy
The therapy continues to be refined, with directions for the future being:
- Off-the-shelf CAR T-cells (allogeneic) – No requirement to work with patient’s own cells
- CAR-NK cell therapy – Employing natural killer cells rather than T-cells
- Combination therapies – With chemo or immune checkpoint inhibitors
- Solid tumor applications – Such as brain, lung, and breast cancer
- CRISPR-edited CAR T-cells – For exact genetic regulation
Summary
CAR T-cell therapy is a revolutionary cancer treatment that reprograms the patient’s own immune system to identify and destroy cancer. It functions by:
- Isolating T-cells
- Editing them to recognize cancer
doubling them in the laboratory - Reinfusing them to locate and destroy cancer cells
Although it can produce serious side effects, it has also brought life-saving remissions for numerous patients who otherwise had no hope. As the study goes on, CAR T-cell therapy is likely to extend to more cancer types and be safer, faster, and more accessible.

