The Role of Mass Spectroscopy in Diagnosing Amyloidosis: Accurate Protein Identification
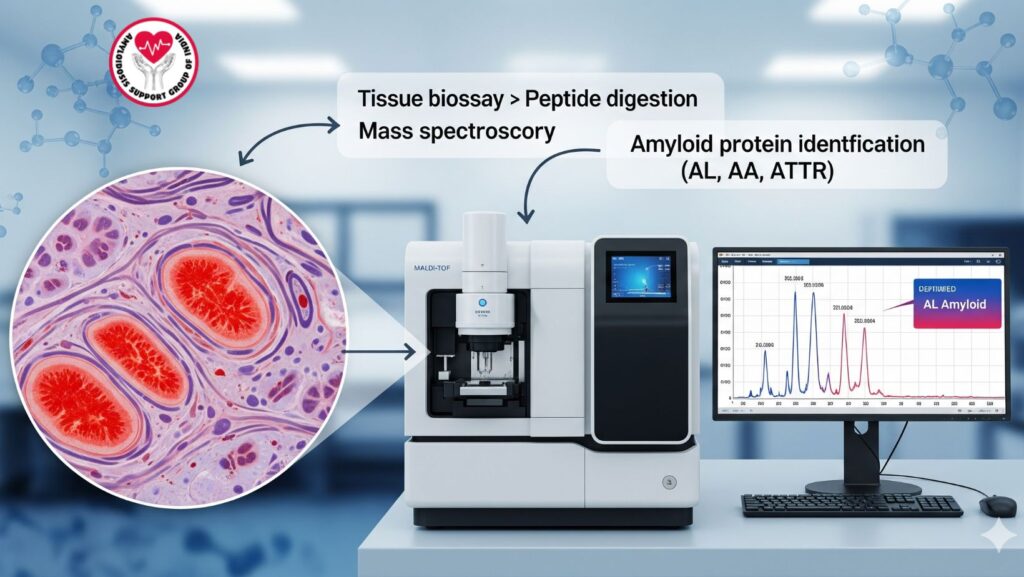
Table of Contents
Introduction
Amyloidosis is a multisystemic condition that involves the presence of atypical amyloid fibrils in organs. Proper diagnosis, in addition to the detection of amyloid deposits, entails proper identification of the specific amyloid protein, which is critical for proper therapy and prognosis.
Mass spectroscopy has become a gold-standard diagnostic test, giving accurate peptide profiling to distinguish between AL (light-chain), AA (serum amyloid A), ATTR (transthyretin), and other amyloids.
This article discusses how mass spectroscopy functions, its diagnostic role in amyloidosis, clinical uses, benefits over classical methods, and real-life case examples.
1. Overview of Amyloidosis
Amyloidosis can be categorized on the basis of protein type and etiology:
- AL (Primary) Amyloidosis
- Due to deposition of immunoglobulin light chains
- Frequent association with plasma cell dyscrasias
- AA (Secondary) Amyloidosis
- Originates from serum amyloid A protein
- Seen in chronic inflammatory diseases
- ATTR (Transthyretin) Amyloidosis
- Due to transthyretin protein misfolding
- Both hereditary and age-related
- Other Rare Forms
- β2-microglobulin in dialysis-related amyloidosis
- Other localized amyloidoses
Accurate typing is crucial since treatment approaches vary greatly between these types.
2. Conventional Diagnostic Techniques and Their Shortcomings
2.1 Congo Red Staining
- Identifies amyloid deposits in tissue biopsies
- Exhibits apple-green birefringence under polarized light
- Shortcoming: Does not determine amyloid type
2.2 Immunohistochemistry (IHC)
- Detects specific amyloid proteins using antibodies
- Shortcomings:
- Cross-reactivity and false negatives
- Needs high-quality tissue
- Can’t always distinguish AL from other forms
2.3 Genetic Testing
- Diagnoses hereditary ATTR amyloidosis
- Shortcoming: Useful only for certain mutations, not all amyloid forms
Conclusion: Conventional tests identify amyloid presence but lack accuracy in typing, which is essential for tailored therapy.
3. Mass Spectroscopy: Principles and Technology
(MS) is a method of analysis that measures the mass-to-charge ratio of ions to detect molecules. In amyloidosis, it is applied for peptide profiling.
3.1 How It Works
- Tissue biopsy with amyloid deposits is broken down into peptides.
- Peptides are ionized and sorted according to mass-to-charge ratio.
- The obtained spectral pattern is matched with known protein databases.
- Software recognizes the specific amyloid protein type with high sensitivity.
3.2 Types of Mass Spectroscopy Utilized
- MALDI-TOF (Matrix-Assisted Laser Desorption Ionization – Time of Flight)
- LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry)
Clinical Implication: These methods allow for accurate amyloid typing, even for complicated or mixed deposits.
4. Role of Mass Spectroscopy in Amyloidosis Diagnosis
- Definitive Amyloid Typing
- Seperates AL, AA, ATTR, and uncommon amyloidoses
- Directs therapy choice (e.g., chemotherapy for AL, tafamidis for ATTR)
- Identification of Mixed Amyloid Deposits
- Certain patients carry multiple amyloid types
- MS can identify multiple proteins in parallel
- Complementary to Histology
- Sequesters amyloid presence and type following Congo red staining
- Clarifies inconclusive or ambiguous IHC findings
- Personalized Therapy Guidance
- Supports targeted treatment according to amyloid type
- Enhances the outcomes of patients and minimizes unnecessary interventions
5. Benefits of Mass Spectroscopy over Conventional Techniques
| Feature | Traditional Methods | Mass Spectroscopy |
| ——————————- | ——————————— | —————————— |
| Protein typing | Limited, may be inaccurate | Highly precise |
| Sensitivity | Can overlook minor amyloid constituents | Can identify low-abundance proteins |
| Detection of mixed amyloidosis | Challenging | Readily identified |
| Tissue requirement | Larger sample needed | Small biopsy sufficient |
| Reproducibility | Variable | Highly reproducible |
Key Point: MS is more reliable, sensitive, and informative, and thus the method of choice in contemporary amyloidosis centers.
6. Clinical Workflow: Incorporating Mass Spectroscopy
Step 1: Biopsy and Congo Red Staining
- Detection of amyloid deposits in diseased tissue
Step 2: Sample Preparation for MS
- Microdissection or laser capture of areas rich in amyloid
- Proteolytic digestion into peptides
Step 3: Mass Spectrometry Analysis - Peptide ions are measured using MALDI-TOF or LC-MS/MS
- Spectral data vs. protein databases
Step 4: Amyloid Protein Typing - Results identify specific amyloid type
- Directs therapy planning and prognosis estimation
7. Case Example: Mass Spectroscopy in AL Amyloidosis
Patient: 58-year-old male with renal and cardiac involvement
Traditional Workup:
- Congo red staining: positive amyloid deposits
- Immunohistochemistry: inconclusive
MS Findings: - Peptide profile identified lambda light chain (AL amyloidosis)
- Therapy: Bortezomib-based chemotherapy begun
- Outcome: Organ function stabilized with decreased amyloid deposition
Clinical Relevance: MS offered definitive protein typing, allowing for prompt and proper treatment.
8. Mass Spectroscopy in ATTR and AA Amyloidosis
*
- ATTR Amyloidosis: Differentiates wild-type from mutant transthyretin
- AA Amyloidosis: Establishes serum amyloid A protein as the deposit
- Benefit: Prevents misdiagnosis and inappropriate therapy
9. Limitations and Challenges of Mass Spectroscopy
*
- Demands specialized equipment and expertise
- May be more expensive than conventional techniques
- Careful tissue preparation is required to prevent contamination
- Not available in all clinical facilities everywhere
Solution: Central reference laboratories typically offer MS-based amyloid typing for greater availability.
10. Emerging Applications
- Quantitative MS: Quantifies amyloid burden for monitoring therapy
- Proteomic profiling: Anticipates organ-specific deposition patterns
- Integration with imaging: Integrates histology, MS, and imaging for global disease evaluation
Impact: Facilitates precision medicine in systemic amyloidosis.
11. Clinical Pearls
- Mass spectroscopy is the reference standard for identification of amyloid protein
- Early and accurate typing directs therapy and optimizes prognosis
- Helpful in doubtful cases or mixed amyloid deposits
- Supplemental to histology, imaging, and laboratory tests
12. Summary Table: Mass Spectroscopy in Amyloidosis
| Feature | Clinical Significance |
| —————————- | ———————————————- |
| Protein identification | Confirms AL, AA, ATTR, or rare amyloids |
| Mixed deposits | Detects multiple amyloid types |
| Low tissue demand | Allows for minimally invasive biopsy |
| Directs proper therapy | Facilitates individualized treatment planning |
| Enhances prognosis | Reduced complications through early accurate diagnosis |
13. Conclusion
Mass spectroscopy has revolutionized the diagnosis of amyloidosis, offering accurate amyloid protein typing by means of peptide profiling. Its benefits are:
- High specificity and sensitivity
- Can identify mixed deposits
- Aid in personalized therapy
- Enhanced patient outcomes and prognosis
Key Takeaways:
- Treatment for amyloidosis relies on correct identification of proteins.
- Mass spectroscopy is more precise than conventional immunohistochemistry and histology.
- Early use in diagnosis allows for improved therapy and improved survival rates.
- Integration with imaging and laboratory data gives a comprehensive assessment of systemic involvement.

