Challenges of Guideline-Directed Medical Therapy in Cardiac Amyloidosis
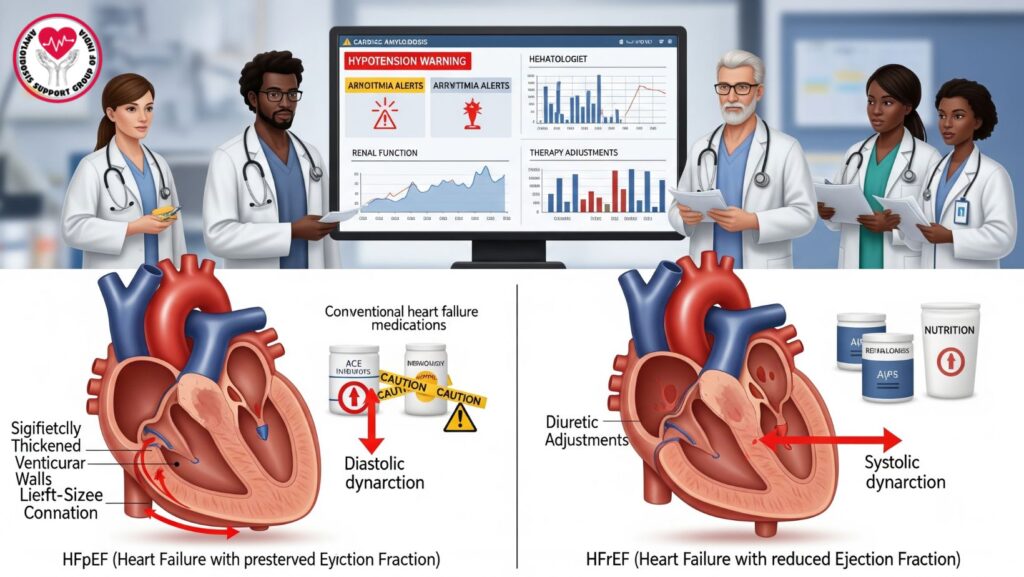
Table of Contents
1. Introduction
Cardiac amyloidosis (CA) is a severe form of systemic amyloidosis, resultant from amyloid protein infiltration of the myocardium. This causes restrictive cardiomyopathy, diastolic dysfunction, arrhythmias, and progressive heart failure.
Guideline-directed medical therapy (GDMT) for heart failure, such as ACE inhibitors, beta-blockers, ARBs, mineralocorticoid receptor antagonists, and SGLT2 inhibitors, is routine in general HF patients. In CA, however, these traditional therapies are frequently not well tolerated, particularly in severe disease.
The difficulty of GDMT in CA and other options for management, together with the role of multidisciplinary care in achieving the best patient outcomes, are discussed in this article.
2. Pathophysiology of Heart Failure in Cardiac Amyloidosis
Amyloid fibrils, especially ALS or ATTR types, accumulate in the myocardium to cause:
- Thickening of the ventricular wall
- Decreased compliance of the myocardium → diastolic dysfunction
- Impaired systolic function in severe disease
- Abnormalities of conduction → arrhythmias
Implications for GDMT: - Decreased ventricular compliance → hypotension during vasodilators
- Arrhythmias may be exacerbated by beta-blockers
- Renal impairment makes ACE inhibitor/ARB use difficult
3. Why GDMT is Often Poorly Tolerated
3.1 Hypotension
- Amyloid infiltration leads to stiff ventricles and reduced stroke volume, making patients prone to hypotension.
- Vasodilators such as ACE inhibitors and ARBs can predispose to hypotension, restricting dose titration.
3.2 Bradyarrhythmias and Heart Block
- Beta-blockers can exacerbate conduction delay, syncope, and fatigue.
- Patients with advanced CA usually develop pre-existing AV block, making beta-blockers unsafe.
3.3 Renal Dysfunction
- Amyloid deposition can affect kidneys → proteinuria, nephrotic syndrome, chronic kidney disease
- ACE inhibitors, ARBs, and diuretics can induce worsening renal function
3.4 Diuretic Dependency
- Looped diuretics are the usual drugs on which patients become dependent to manage fluid balance but which worsen hypotension and renal injury and complicate the use of GDMT.
3.5 Limited Evidence Base
- The majority of GDMT trials omit patients with CA, hence limited efficacy and safety data
- Doctors have to fall back on case reports and expert opinion
4. Heart Failure Phenotypes in CA and Tolerance to GDMT
4.1 HFpEF (Heart Failure with Preserved EF)
- Most prevalent phenotype in CA
- Diastolic dysfunction prevails, EF maintained
- GDMT restricted because patients are preload-dependent, and vasodilators and beta-blockers are hazardous
4.2 HFrEF (Heart Failure with Decreased EF)
- Less frequent, with extensive amyloid deposition
- GDMT can be partly tolerated, but hypotension and renal dysfunction still restrict use
5. Alternative and Supportive Management Strategies
5.1 Diuretics
- Loop diuretics for fluid overload
- Thiazides can be used in combination
- Titrated carefully to support renal function and blood pressure
5.2 Rate and Rhythm Control
- Amiodarone used first line for AF/flutter
- Pacemakers for bradyarrhythmias or AV block
- Avoid aggressive beta-blockers unless well tolerated
5.3 Disease-Specific Therapy
- AL amyloidosis: Chemotherapy, proteasome inhibitors, stem cell transplantation
- ATTR amyloidosis: Tafamidis, diflunisal, RNA therapies
- Reducing amyloid load can enhance cardiac function and tolerance to supportive therapies
5.4 Advanced Heart Failure Therapies
- Heart transplant reserved for selected cases
- Mechanical circulatory support limited by small, stiff ventricles
5.5 Multidisciplinary Supportive Care
- Coordination among cardiology, nephrology, hematology, nutrition, and palliative care
- Dietary changes, physical rehabilitation, and symptom control enhance quality of life
6. Monitoring and Safety Considerations
*
- Regular blood pressure and renal function checks
- Regular monitoring of electrolytes on diuretic therapy
- ECG for arrhythmias or conduction delay
- Biomarkers (BNP, troponin) direct therapy intensity
7. Patient Education and Lifestyle
- Minimize sudden postural changes to avoid hypotension
- Daily weight monitoring for fluid status
- Compliance with low-salt diet
- Recognition of early signs of fluid overload or arrhythmia
- Participate in mild, supervised exercise
8. Emerging Therapies and Research Directions
- Gene-silencing therapy (patisiran, inotersen) for ATTR amyloidosis
- New proteasome inhibitors and monoclonal antibodies for AL amyloidosis
- Combining GDMT with disease-specific therapy to enhance tolerability
- Telemonitoring and digital health solutions for hemodynamic stability
9. Prognosis and Outcomes
- Poor GDMT tolerance leads to adverse outcomes in advanced CA
- Early diagnosis and treatment with disease-specific therapy enhances prognosis
- Multidisciplinary supportive care is vital for symptom control and quality of life
10. Case Example (Optional)
A 68-year-old patient with AL cardiac amyloidosis:
- Developed HFpEF with severe hypotension
- ACE inhibitors and beta-blockers were not tolerated
- Treated with loop diuretics, meticulous fluid management, and amiodarone for atrial fibrillation
- Treated with chemotherapy for the reduction of light-chain production
- Outcome: Stabilized cardiac symptoms, better functional capacity, and delayed deterioration to HFrEF
11. Conclusion
Guideline-directed medical therapy, though normative for typical heart failure, is frequently poorly tolerated in cardiac amyloidosis, particularly in severe disease with HFpEF or HFrEF.
Key Takeaways:
- Limitations of GDMT are a consequence of hypotension, bradyarrhythmias, renal impairment, and fluid dependence
- Disease-specific treatment is still crucial for enhancing cardiac function
- Multidisciplinary supportive care maximizes survival and quality of life
- Individualized treatment plan and close monitoring are important

