Pharmacologic Treatment of Gastric Symptoms in Gastrointestinal Amyloidosis
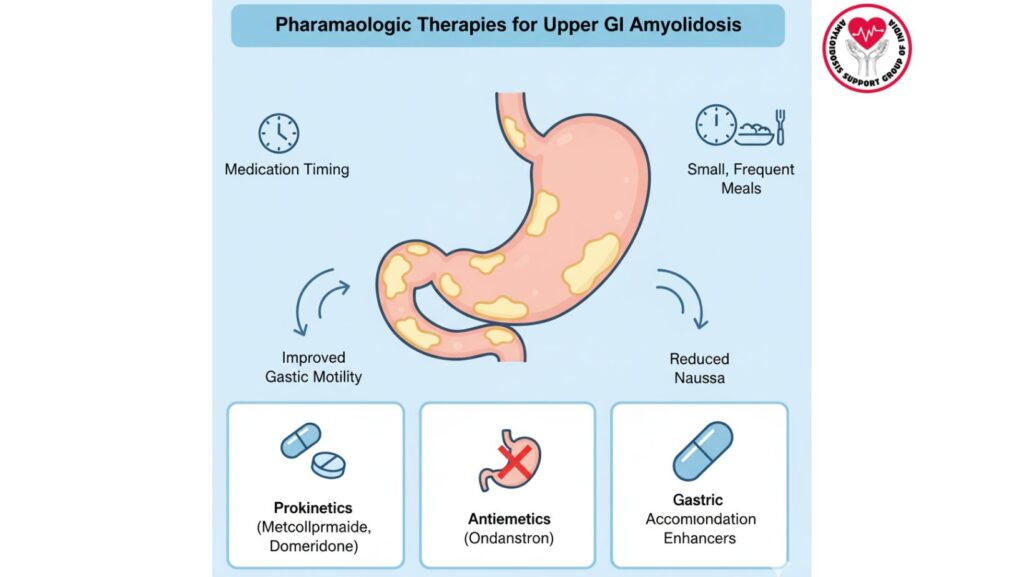
Table of Contents
Introduction
Gastrointestinal (GI) amyloidosis is a manifestation of systemic amyloidosis that involves the stomach, small intestine, and colon and commonly results in early satiety, nausea, vomiting, bloating, and abdominal pain.
Pharmacologic treatment is indicated when dietary and lifestyle changes are not enough to control gastric symptoms. Prokinetic agents, gastric accommodation enhancers, and antiemetics are often employed to enhance gastric motility, decrease nausea, and improve overall quality of life.
This article is a comprehensive overview of pharmacologic options, mechanisms, clinical application, dosing, monitoring, and emerging therapies for the treatment of gastric symptoms in amyloidosis patients.
1. Pathophysiology of Gastric Symptoms in Amyloidosis
1.1 Amyloid Deposition
- Amyloid proteins accumulate in the stomach wall, submucosa, and autonomic nerves.
- Interferes with normal gastric motility and accommodation, leading to early satiety, nausea, and delayed gastric emptying.
1.2 Autonomic Neuropathy
- Amyloid infiltration of enteric nerves leads to disturbed gastric signaling.
- Results in gastroparesis, dyspepsia, and impaired gastric emptying, even in the absence of mechanical obstruction.
1.3 Clinical Implications
- Symptoms can markedly compromise nutrition, hydration, and quality of life.
- Pharmacologic treatment is frequently necessary in addition to dietary modifications to ensure caloric intake.
2. Typical Gastric Symptoms of GI Amyloidosis
- Early satiety: fullness after minimal meals
- Nausea and vomiting: frequently exacerbated by delayed gastric emptying
- Bloating and abdominal pain
- Postprandial fullness
- Decreased appetite resulting in weight loss
Identification of these symptoms is necessary to begin proper pharmacologic treatment.
3. Overview of Pharmacologic Therapy
3.1 Objectives
- Enhance gastric emptying
- Decrease nausea and vomiting
- Increase gastric accommodation
- Improve oral intake and nutrition
3.2 Main Classes of Drugs
- Prokinetic agents: increase gastric motility
- Gastric accommodation enhancers: increase gastric relaxation and capacity
- Antiemetics: decrease nausea and vomiting
4. Prokinetic Agents
4.1 Mechanism of Action
- Stimulate gastric smooth muscle contraction
- Enhance coordination of gastric emptying
- Decrease symptoms of gastroparesis and postprandial fullness
4.2 Common Prokinetics
4.2.1 Metoclopramide
- Dopamine D2 receptor antagonist
- Increases gastric motility and esophageal peristalsis
- Decreases nausea and vomiting
Dosage and Administration: - 10 mg orally 30 minutes prior to meals, up to 4 times a day
- IV formulation in severe cases
Side Effects: - Extrapyramidal symptoms (tremor, dystonia)
- Tardive dyskinesia with chronic use
- Sedation and diarrhea
4.2.2 Domperidone
- Peripheral D2 receptor blocker
- Increases gastric motility with minimal CNS penetration
Dosage: - 10–20 mg orally 3–4 times a day, 15–30 minutes prior to meals
- Frequently used in patients at risk for CNS side effects
Side Effects: - QT prolongation (ECG monitoring is advisable)
- Mild gastrointestinal discomfort
- Infrequent cardiac arrhythmias
4.3 Clinical Considerations
- Begin low dose and gradually titrate
- Watch for neurologic and cardiac side effects
- Use in conjunction with dietary adjustments for maximum effect
5. Gastric Accommodation Enhancers
5.1 Mechanism
- Enhance fundic relaxation and gastric volume
- Minimize early satiety and postprandial fullness
5.2 Common Agents
- Buspirone (5-HT1A agonist): increases gastric accommodation
- Acotiamide (where available): enhances fundic relaxation and gastric motility
5.3 Clinical Use
- Very useful in early satiety-dominant symptoms
- Frequently combined with prokinetics for synergism
6. Antiemetic Therapy
6.1 Mechanism
- Inhibit central and peripheral pathways initiating nausea
- Enhance patient comfort and oral intake
6.2 Common Antiemetics
- Ondansetron: 5-HT3 receptor antagonist
- Domperidone/metoclopramide: both have antiemetic properties
- Prochlorperazine: dopamine antagonist
- Ginger supplements (adjunctive)
6.3 Clinical Considerations
- Choose based on mechanism of action and patient comorbidities
- Watch for QT prolongation, sedation, or extrapyramidal symptoms
7. Combination Therapy
- Most patients derive benefit from combining prokinetics and antiemetics
- Supplement with gastric accommodation enhancers if early satiety continues
- Dietary modifications (small, frequent meals, low-fat diet) increase efficacy
8. Monitoring and Safety
- Cardiac monitoring for QT prolongation with domperidone or ondansetron
- Neurologic monitoring for extrapyramidal symptoms due to metoclopramide
- Check weight, nutritional intake, and improvement in symptoms
- Titrate therapy on tolerance and response
9. Special Populations
9.1 Elderly Patients
- Increased risk of CNS and cardiac side effects
- Initiate at lower doses and escalate cautiously
9.2 Patients with Cardiac Amyloidosis
- Preferred use of antiemetics and prokinetics to preclude QT prolongation
- Monitor fluid status and cardiac function
9.3 Renal Impairment
- Dose adjust metoclopramide and domperidone if renal function decreased
10. Case Examples
Case 1: Gastroparesis-Predominant Symptoms
- 62-year-old patient with AL amyloidosis and nausea, early satiety
- Intervention: Metoclopramide 10 mg TID + small, frequent meals
- Outcome: Improved gastric emptying, decreased nausea, improved caloric intake
Case 2: Early Satiety and Postprandial Fullness
- 58-year-old ATTR patient
- Intervention: Buspirone 10 mg TID + dietary changes
- Outcome: Improved meal tolerance, weight stabilization
11. Emerging Therapies
- New prokinetics in development: ghrelin agonists, motilin agonists
- Targeted therapies for reducing amyloid can indirectly benefit gastric symptoms
- Neuromodulation and gastric electrical stimulation for resistant gastroparesis
12. Integrating Pharmacologic Therapy with Multidisciplinary Care
- Dietitians: optimize meal and caloric ingestion
- Gastroenterologists: follow symptom response and medication titration
- Hematologists or amyloidosis specialists: manage systemic therapy
- Primary care providers: coordinate overall safety, follow comorbidities
13. Patient Education
- Educate on timing of medications with meals
- Warn about possible side effects: extrapyramidal symptoms, sedation, QT prolongation
- Encourage tracking symptom improvement
- Combine with dietary and hydration strategies for best outcomes
14. Conclusion
Gastric symptoms in gastrointestinal amyloidosis have major effects on nutrition, hydration, and quality of life. Pharmacologic treatment, including prokinetics, gastric accommodation enhancers, and antiemetics, is the centerpiece of symptom control.
Key Points:
- Prokinetics enhance gastric motility and alleviate nausea
- Gastric accommodation enhancers assist in early satiety
- Antiemetics suppress nausea and vomiting
- Combination therapy along with dietary modifications is usually most effective
- Watch for side effects and adjust therapy according to individual patient requirement
A multidisciplinary approach, which involves dietary management, symptom management, and systemic amyloidosis treatment, provides the best outcomes for patients with gastric complications of amyloidosis.

