Diagnosing AL Amyloidosis Through Sigmoid Biopsy – Role of Congo Red Staining and Mass Spectroscopy
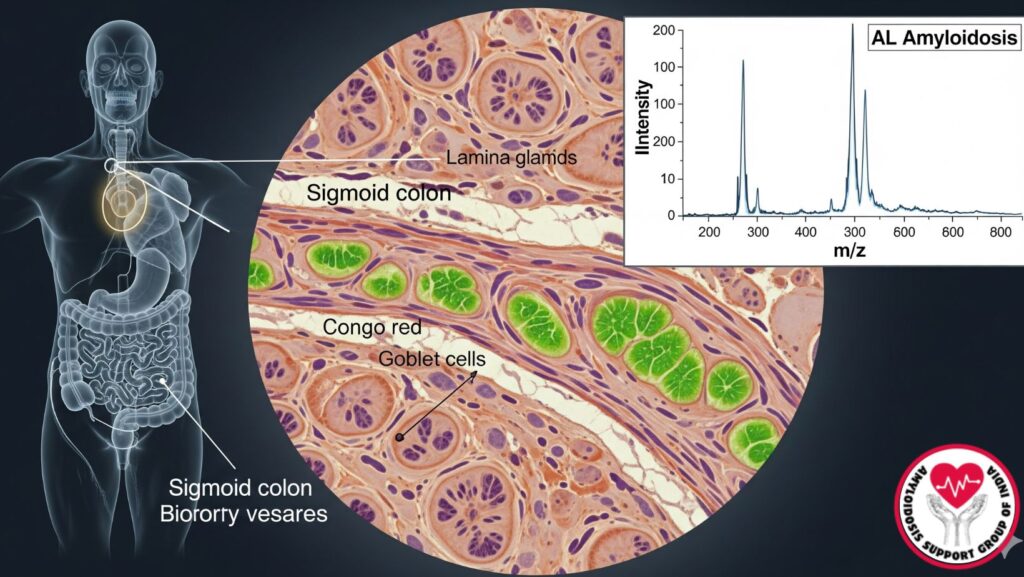
Table of Contents
Introduction
AL (primary) amyloidosis is an uncommon systemic disease that involves the deposition of misfolded immunoglobulin light chains as amyloid fibrils in a variety of organs, including the heart, kidneys, liver, and gastrointestinal tract. Prompt and precise diagnosis is essential for management and prognosis, but diagnosis is frequently difficult because of the heterogeneous clinical presentation.
Gastrointestinal biopsies, such as the sigmoid colon, have an important role in the detection of amyloid deposits. Congo red staining and mass spectroscopy are the standard tests for the diagnosis of AL amyloidosis and typing of amyloid.
This inclusive guide discusses:
- Pathophysiology and clinical significance of AL amyloidosis
- Gastrointestinal biopsies in diagnosis
- Methods of Congo red staining and mass spectroscopy
- Case presentations and clinical use
- Prognostic significance and future research directions
Epidemiology of AL Amyloidosis
Systemic Involvement
- AL amyloidosis occurs in 3–9 per million individuals per year.
- Multi-organ involvement is frequent:
- Kidneys: ~70% (nephrotic-range proteinuria)
- Heart: ~50% (cardiomyopathy, arrhythmias)
- Liver: 60–90% (usually mild, seldom liver failure)
- Gastrointestinal tract: 10–15%
Demographics
- Usually presents in older adults (55–70 years).
- Mild male predominance in some reports.
Pathophysiology
- AL amyloidosis is due to monoclonal plasma cell dyscrasias, resulting in aberrant light chains that misfold.
- Misfolded proteins form amyloid fibrils, depositing outside cells.
- Tissue deposition causes organ dysfunction, varying with site and degree.
Gastrointestinal Involvement
- Amyloid may deposit in muscularis mucosa, submucosa, and blood vessels.
- Typical findings: malabsorption, bleeding, disorders of motility, diarrhea, and very infrequently bowel ischemia.
- Biopsy of involved sites, e.g., sigmoid colon, is definitive.
Clinical Indications for Sigmoid Biopsy
When to Consider GI Biopsy
- Unexplained gastrointestinal symptoms in patients with presumed AL amyloidosis
- Finding of multi-organ amyloidosis (renal, cardiac, hepatic)
- Unexplained proteinuria or liver dysfunction
- Situations in which less invasive biopsies (fat pad, bone marrow) are unsatisfactory
Advantages of Sigmoid Biopsy
- Can be reached through colonoscopy
- Offers adequate tissue for mass spectroscopy and histopathology
- Can identify systemic amyloidosis even at subclinical levels
Histopathologic Diagnosis
Congo Red Staining
- Gold standard for the detection of amyloid.
- Sections of tissue stained with Congo red show apple-green birefringence when viewed under polarized light.
- Strengths:
- Specific and sensitive for amyloid
- Visual identification of deposition patterns
- Weaknesses:
- Can’t identify amyloid type (AL vs AA)
- Needs sufficient tissue sample
Mass Spectroscopy
- Applied for amyloid typing following Congo red positivity.
- Laser microdissection separates amyloid deposits.
- Mass spectrometry detects characteristic peptide profiles, establishing AL (light chain) amyloidosis.
- Benefits:
- Extremely specific
- Distinguishes AL amyloidosis from hereditary or AA amyloidosis
- Directs therapy choice
Complementary Diagnostic Tests
*
- Fat pad aspiration: Less risky, but decreased sensitivity (~70%).
- Bone marrow biopsy: Finds plasma cell dyscrasia.
- Serum and urine protein electrophoresis: Finds monoclonal proteins.
- Free light chain assay: Quantitates kappa and lambda chains, critical for diagnosis and follow-up.
- Imaging: Echocardiogram, MRI, CT, or MRI elastography for organ involvement.
Case Study Example
Patient Profile:
- 61-year-old male with ascites, lower extremity edema, and mild jaundice.
- Renal involvement: Nephrotic-range proteinuria.
- Liver involvement: Slightly raised ALP, AST, ALT.
Diagnostic Workup: - Sigmoid biopsy done because of unexplained proteinuria and GI symptoms.
- Congo red staining: amyloid deposition positive.
- Mass spectroscopy: Peptide profile as in primary AL amyloidosis.
Outcome: - Multi-organ AL amyloidosis established.
- Therapeutic limitations secondary to hepatic impairment.
- Poor prognosis because of multi-organ involvement and liver function compromise.
Clinical Significance
*
- Sigmoid biopsy is a definitive diagnosis when less invasive biopsies are nondiagnostic.
- Early diagnosis of AL amyloidosis enables:
- Prompt chemotherapy initiation (bortezomib, cyclophosphamide, dexamethasone)
- Evaluation of organ involvement and treatment planning
- Prevention of misdiagnosis and incorrect therapies
Benefits of Congo Red and Mass Spectroscopy Combined
- Congo red: Positive diagnosis of amyloid
- Mass spectroscopy: Identifies amyloid type (AL, AA, hereditary)
- Combined strategy: Necessary for correct diagnosis and treatment decisions
Prognostic Significance
*
- Amyloid type and organ involvement directly affect prognosis.
- AL amyloidosis with GI and hepatic involvement carries increased mortality risk.
- Median survival:
- In the absence of significant organ involvement: 2–3 years
- Multi-organ involvement including failure of the liver: <6 months
Considerations in Management
Treatment Plans
- Chemotherapy: Bortezomib-based regimens routine, but liver function could preclude use.
- Supportive care: Treat ascites, diarrhea, and nutritional deficiencies.
- Organ-specific monitoring: Renal, cardiac, and hepatic parameters.
Role of Early Diagnosis
- Enables early intervention prior to multi-organ failure.
- Enables risk stratification and transplant or palliative care planning.
Future Directions
The future directions for AL amyloidosis are outlined as follows:
- Non-invasive amyloid detection: Imaging methods and biomarkers
- Advanced mass spectrometry: More rapid and accurate typing
- Targeted therapies: Light chain stabilizers, monoclonal antibodies to amyloid fibrils
- Personalized medicine: Treatment tailored depending on amyloid type and organs involved
Clinical Pearls
Sigmoid biopsy* is useful in unexplained GI symptoms or suspicion of systemic amyloidosis.
- Congo red staining is still the gold standard for amyloid diagnosis.
- Mass spectroscopy is critical for AL amyloidosis typing and therapy.
- Early diagnosis enhances patient management, even when prognosis relies on multi-organ involvement.
- Multidisciplinary approach is needed for best results.
Conclusion
Diagnosis of AL amyloidosis may be tricky owing to its variable clinical presentation. Gastrointestinal biopsies, especially sigmoid biopsies, with Congo red staining and mass spectroscopy offer a definitive diagnosis.
Key points:
- Early diagnosis is vital to initiate timely intervention and plan treatment.
- Involvement of multiple organs, particularly the liver and heart, influences prognosis and management.
- Multidisciplinary input from hematology, gastroenterology, cardiology, and nephrology is required.
Sigmoid biopsy using sophisticated diagnostic methods is an essential tool for the precise diagnosis of AL amyloidosis, allowing doctors to make informed choices and enhance patient outcomes.

